Selectin-Mediated Signaling-Shedding Light on the Regulation of Integrin Activity in Neutrophils
- PMID: 35455989
- PMCID: PMC9025114
- DOI: 10.3390/cells11081310
Selectin-Mediated Signaling-Shedding Light on the Regulation of Integrin Activity in Neutrophils
Abstract
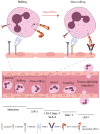
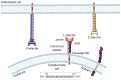
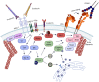
As a consequence of tissue injury or infection, neutrophils are recruited in a stepwise recruitment process from the bloodstream into the surrounding tissue. Selectins are a family of adhesion molecules comprised of L-, E-, and P-selectin. Differences in expression patterns, protein structure, and ligand binding characteristics mediate distinct functions of each selectin. Interactions of selectins and their counter-receptors mediate the first contact of neutrophils with the endothelium, as well as subsequent neutrophil rolling along the endothelial surface. For efficient neutrophil recruitment, activation of β2-integrins on the cell surface is essential. Integrin activation can be elicited via selectin- as well as chemokine-mediated inside-out signaling resulting in integrin conformational changes and clustering. Dysregulation of selectin-induced integrin activation on neutrophils is involved in the development of severe pathological disease conditions including leukocyte adhesion deficiency (LAD) syndromes in humans. Here, we review molecular mechanisms involved in selectin-mediated signaling pathways in neutrophils and their impact on integrin activation, neutrophil recruitment, and inflammatory diseases.
Keywords: PSGL-1; integrin; leukocyte recruitment; neutrophil; selectin; shedding; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

