CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts
- PMID: 35455995
- PMCID: PMC9031661
- DOI: 10.3390/cells11081316
CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts
Abstract
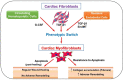
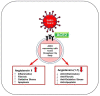
Cardiovascular disease (CVD) is the leading cause of death worldwide. Current data suggest that patients with cardiovascular diseases experience more serious complications with coronavirus disease-19 (COVID-19) than those without CVD. In addition, severe COVID-19 appears to cause acute cardiac injury, as well as long-term adverse remodeling of heart tissue. Cardiac fibroblasts and myofibroblasts, being crucial in response to injury, may play a pivotal role in both contributing to and healing COVID-19-induced cardiac injury. The role of cardiac myofibroblasts in cardiac fibrosis has been well-established in the literature for decades. However, with the emergence of the novel coronavirus SARS-CoV-2, new cardiac complications are arising. Bursts of inflammatory cytokines and upregulation of TGF-β1 and angiotensin (AngII) are common in severe COVID-19 patients. Cytokines, TGF-β1, and Ang II can induce cardiac fibroblast differentiation, potentially leading to fibrosis. This review details the key information concerning the role of cardiac myofibroblasts in CVD and COVID-19 complications. Additionally, new factors including controlling ACE2 expression and microRNA regulation are explored as promising treatments for both COVID-19 and CVD. Further understanding of this topic may provide insight into the long-term cardiac manifestations of the COVID-19 pandemic and ways to mitigate its negative effects.
Keywords: COVID-19; TGF-β1; fibroblast; fibrosis; myofibroblast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.Exp Biol Med (Maywood). 2018 Apr;243(7):601-612. doi: 10.1177/1535370218761628. Epub 2018 Mar 4. Exp Biol Med (Maywood). 2018. PMID: 29504479 Free PMC article.
-
Angiotensin II upregulates fibroblast-myofibroblast transition through Cx43-dependent CaMKII and TGF-β1 signaling in neonatal rat cardiac fibroblasts.Acta Biochim Biophys Sin (Shanghai). 2018 Sep 1;50(9):843-852. doi: 10.1093/abbs/gmy090. Acta Biochim Biophys Sin (Shanghai). 2018. PMID: 30060053
-
Krüppel-like factor 4 transcriptionally regulates TGF-β1 and contributes to cardiac myofibroblast differentiation.PLoS One. 2013 Apr 30;8(4):e63424. doi: 10.1371/journal.pone.0063424. Print 2013. PLoS One. 2013. PMID: 23646205 Free PMC article.
-
Biomarkers for the identification of cardiac fibroblast and myofibroblast cells.Heart Fail Rev. 2019 Jan;24(1):1-15. doi: 10.1007/s10741-018-9720-1. Heart Fail Rev. 2019. PMID: 29987445 Review.
-
Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages.Curr Pharm Des. 2007;13(12):1247-56. doi: 10.2174/138161207780618885. Curr Pharm Des. 2007. PMID: 17504233 Review.
Cited by
-
Rutin and quercetagetin enhance the regeneration potential of young and aging bone marrow-derived mesenchymal stem cells in the rat infarcted myocardium.Mol Cell Biochem. 2023 Aug;478(8):1759-1770. doi: 10.1007/s11010-022-04628-5. Epub 2022 Dec 25. Mol Cell Biochem. 2023. PMID: 36566485
-
Effects of COVID-19 on the cardiovascular system: A mendelian randomization study.Sports Med Health Sci. 2024 Jun 4;6(3):266-272. doi: 10.1016/j.smhs.2024.06.001. eCollection 2024 Sep. Sports Med Health Sci. 2024. PMID: 39234491 Free PMC article.
-
Myocardial Oedema as a Consequence of Viral Infection and Persistence-A Narrative Review with Focus on COVID-19 and Post COVID Sequelae.Viruses. 2024 Jan 14;16(1):121. doi: 10.3390/v16010121. Viruses. 2024. PMID: 38257821 Free PMC article. Review.
-
Interaction of COVID-19 With Common Cardiovascular Disorders.Circ Res. 2023 May 12;132(10):1259-1271. doi: 10.1161/CIRCRESAHA.122.321952. Epub 2023 May 11. Circ Res. 2023. PMID: 37167359 Free PMC article. Review.
-
The Eye of the Storm: Investigating the Long-Term Cardiovascular Effects of COVID-19 and Variants.Cells. 2023 Aug 27;12(17):2154. doi: 10.3390/cells12172154. Cells. 2023. PMID: 37681886 Free PMC article. Review.
References
-
- World Health Organization Cardiovascular Diseases (CVDs) [(accessed on 16 December 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...
-
- Moody W.E., Liu B., Mahmoud-Elsayed H.M., Senior J., Lalla S.S., Khan-Kheil A.M., Brown S., Saif A., Moss A., Bradlow W.M., et al. Persisting Adverse Ventricular Remodeling in COVID-19 Survivors: A Longitudinal Echocardiographic Study. J. Am. Soc. Echocardiogr. 2021;34:562–566. doi: 10.1016/j.echo.2021.01.020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

