Secretory Immunoglobulin A Immunity in Chronic Obstructive Respiratory Diseases
- PMID: 35456002
- PMCID: PMC9027823
- DOI: 10.3390/cells11081324
Secretory Immunoglobulin A Immunity in Chronic Obstructive Respiratory Diseases
Abstract
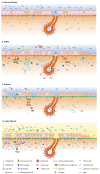
Chronic obstructive pulmonary disease (COPD), asthma and cystic fibrosis (CF) are distinct respiratory diseases that share features such as the obstruction of small airways and disease flare-ups that are called exacerbations and are often caused by infections. Along the airway epithelium, immunoglobulin (Ig) A contributes to first line mucosal protection against inhaled particles and pathogens. Dimeric IgA produced by mucosal plasma cells is transported towards the apical pole of airway epithelial cells by the polymeric Ig receptor (pIgR), where it is released as secretory IgA. Secretory IgA mediates immune exclusion and promotes the clearance of pathogens from the airway surface by inhibiting their adherence to the epithelium. In this review, we summarize the current knowledge regarding alterations of the IgA/pIgR system observed in those major obstructive airway diseases and discuss their implication for disease pathogenesis.
Keywords: COPD; asthma; cystic fibrosis; immunoglobulin A; mucosal immunity; pIgR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., Abbasi-Kangevari M., Abbastabar H., Abd-Allah F., Abdelalim A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

