Role of Estrogens in Menstrual Migraine
- PMID: 35456034
- PMCID: PMC9025552
- DOI: 10.3390/cells11081355
Role of Estrogens in Menstrual Migraine
Abstract
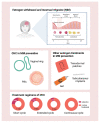
Migraine is a major neurological disorder affecting one in nine adults worldwide with a significant impact on health care and socioeconomic systems. Migraine is more prevalent in women than in men, with 17% of all women meeting the diagnostic criteria for migraine. In women, the frequency of migraine attacks shows variations over the menstrual cycle and pregnancy, and the use of combined hormonal contraception (CHC) or hormone replacement therapy (HRT) can unveil or modify migraine disease. In the general population, 18-25% of female migraineurs display a menstrual association of their headache. Here we present an overview on the evidence supporting the role of reproductive hormones, in particular estrogens, in the pathophysiology of migraine. We also analyze the efficacy and safety of prescribing exogenous estrogens as a potential treatment for menstrual-related migraine. Finally, we point to controversial issues and future research areas in the field of reproductive hormones and migraine.
Keywords: calcitonin gene-related peptide; contraception; efficacy; estradiol; ethinylestradiol; gender; hormone replacement therapy; progesterone; regimen; reproductive hormones.
Conflict of interest statement
R.E.N. had past financial relationships (lecturer, member of advisory boards and/or consultant) with Boehringer Ingelheim, Ely Lilly, Endoceutics, Gedeon Richter, HRA Pharma, Merck Sharpe & Dohme, Procter & Gamble Co., TEVA Women’s Health Inc. and Zambon SpA. At present, she has an ongoing relationship with Astellas, Bayer HealthCare AG, Exceltis, Fidia, Novo Nordisk, Organon & Co., Palatin Technologies, Pfizer Inc., Shionogi Limited and Theramex. S.S. had a financial relationship (lecturer or member of advisory board) with Abbott, Allergan, Novartis, Teva, and Eli Lilly. R.D.I. has received speaker honoraria for lecturing for Eli-Lilly and TEVA. C.T. received honoraria for consultancy on advisory boards for Allergan, ElectroCore, Eli Lilly, Novartis, and Teva and lecturing for Allergan, Eli Lilly, Novartis, and Teva. Her Institute received funding for clinical trials by Alder, Amgen, Eli Lilly, and Teva. She received grants from the European Commission, the Italian Ministry of Health, and the Italian Ministry of University. None of these companies are relevant to the present work. Other authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

