Mucosal Antibody Response to SARS-CoV-2 in Paediatric and Adult Patients: A Longitudinal Study
- PMID: 35456072
- PMCID: PMC9026526
- DOI: 10.3390/pathogens11040397
Mucosal Antibody Response to SARS-CoV-2 in Paediatric and Adult Patients: A Longitudinal Study
Abstract
Background: SARS-CoV-2 enters the body through inhalation or self-inoculation to mucosal surfaces. The kinetics of the ocular and nasal mucosal-specific-immunoglobulin A(IgA) responses remain under-studied.
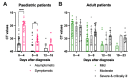
Methods: Conjunctival fluid (CF, n = 140) and nasal epithelial lining fluid (NELF, n = 424) obtained by paper strips and plasma (n = 153) were collected longitudinally from SARS-CoV-2 paediatric (n = 34) and adult (n = 47) patients. The SARS-CoV-2 spike protein 1(S1)-specific mucosal antibody levels in COVID-19 patients, from hospital admission to six months post-diagnosis, were assessed.
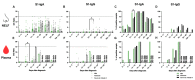
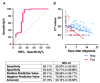
Results: The mucosal antibody was IgA-predominant. In the NELF of asymptomatic paediatric patients, S1-specific IgA was induced as early as the first four days post-diagnosis. Their plasma S1-specific IgG levels were higher than in symptomatic patients in the second week after diagnosis. The IgA and IgG levels correlated positively with the surrogate neutralization readout. The detectable NELF "receptor-blocking" S1-specific IgA in the first week after diagnosis correlated with a rapid decline in viral load.
Conclusions: Early and intense nasal S1-specific IgA levels link to a rapid decrease in viral load. Our results provide insights into the role of mucosal immunity in SARS-CoV-2 exposure and protection. There may be a role of NELF IgA in the screening and diagnosis of SARS-CoV-2 infection.
Keywords: SARS-CoV-2; mucosal antibody; paediatric; specific IgA; specific IgG.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



 and below
and below  the thresholds level are plotted against time. For this panel data, a fixed-effect regression model was applied to compare the changes of the CT values across time between these 2 groups. A statistically significant difference was found in the decline rate of the viral load at p = 0.002.
the thresholds level are plotted against time. For this panel data, a fixed-effect regression model was applied to compare the changes of the CT values across time between these 2 groups. A statistically significant difference was found in the decline rate of the viral load at p = 0.002.References
-
- Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., Talavera-Lopez C., Maatz H., Reichart D., Sampaziotis F., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. - DOI - PMC - PubMed
-
- Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., Raeber M.E., Adamo S., Weigang S., Emmenegger M., et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021;147:545–557.e9. doi: 10.1016/j.jaci.2020.10.040. - DOI - PMC - PubMed
Grants and funding
- PRP/039/21FX/The Innovation and Technology Fund, HKSAR
- COVID190112/Health and Medical Research Fund commissioned grant
- COVID190103/Health and Medical Research Fund commissioned grant
- COVID19F06/Health and Medical Research Fund commissioned grant
- 2020.075/The Chinese University of Hong Kong Direct Grant for Research
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

