Development of a Real-Time Recombinase Polymerase Amplification Assay for the Rapid Detection of African Swine Fever Virus Genotype I and II
- PMID: 35456114
- PMCID: PMC9026452
- DOI: 10.3390/pathogens11040439
Development of a Real-Time Recombinase Polymerase Amplification Assay for the Rapid Detection of African Swine Fever Virus Genotype I and II
Abstract
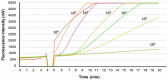
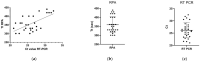
African swine fever (ASF) is a contagious viral disease in pigs and wild boars which poses a major threat to the pig industry. Rapid and accurate diagnosis is necessary to control ASF. Hence, we developed a rapid diagnostic method using a recombinase polymerase amplification (RPA) assay targeting the conserved sequences of CP204L (p30) thatcan rapidly detect ASF virus (ASFV) genotype strains I and II. The lower detection limit of the real-time RPA assay was 5 × 101 copies per reaction. The real-time RPA assay effectively detected ASFV isolates and clinical specimens belonging to ASFV genotypes I and II. The sensitivity and specificity of the assay were 96.8% (95% confidence interval (CI): 83.3−99.9) and 100% (95% CI: 88.4−100.0), respectively. The agreement between the real-time RPA assay and a reference commercial real-time quantitative polymerase chain reaction (qPCR) was 100%. The real-time RPA assay had a detection time of 6.0 min (95% CI: 5.7−6.2), which was significantly shorter than that of qPCR (49 min; 95% CI: 47.4−50.6; p < 0.001). Thus, the developed real-time RPA assay is a rapid and accurate diagnostic tool for detecting ASFV genotypes I and II.
Keywords: African swine fever virus (ASFV); CP204 gene; genotype I; genotype II; recombinase polymerase amplification (RPA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Clinical Validation of Two Recombinase-Based Isothermal Amplification Assays (RPA/RAA) for the Rapid Detection of African Swine Fever Virus.Front Microbiol. 2020 Jul 21;11:1696. doi: 10.3389/fmicb.2020.01696. eCollection 2020. Front Microbiol. 2020. PMID: 32793160 Free PMC article.
-
Development of Real-Time and Lateral Flow Dipstick Recombinase Polymerase Amplification Assays for the Rapid Field Diagnosis of MGF-505R Gene-Deleted Mutants of African Swine Fever Virus.Vet Sci. 2025 Feb 20;12(3):193. doi: 10.3390/vetsci12030193. Vet Sci. 2025. PMID: 40266939 Free PMC article.
-
KP177R-based visual assay integrating RPA and CRISPR/Cas12a for the detection of African swine fever virus.Front Immunol. 2024 Apr 9;15:1358960. doi: 10.3389/fimmu.2024.1358960. eCollection 2024. Front Immunol. 2024. PMID: 38655256 Free PMC article.
-
Rapid Extraction and Detection of African Swine Fever Virus DNA Based on Isothermal Recombinase Polymerase Amplification Assay.Viruses. 2021 Aug 31;13(9):1731. doi: 10.3390/v13091731. Viruses. 2021. PMID: 34578312 Free PMC article.
-
Rapid and Sensitive Recombinase Polymerase Amplification Combined With Lateral Flow Strip for Detecting African Swine Fever Virus.Front Microbiol. 2019 May 15;10:1004. doi: 10.3389/fmicb.2019.01004. eCollection 2019. Front Microbiol. 2019. PMID: 31156571 Free PMC article.
Cited by
-
A triplex crystal digital PCR for the detection of genotypes I and II African swine fever virus.Front Vet Sci. 2024 Apr 2;11:1351596. doi: 10.3389/fvets.2024.1351596. eCollection 2024. Front Vet Sci. 2024. PMID: 38628942 Free PMC article.
-
Advanced Strategies for Developing Vaccines and Diagnostic Tools for African Swine Fever.Viruses. 2023 Oct 28;15(11):2169. doi: 10.3390/v15112169. Viruses. 2023. PMID: 38005846 Free PMC article. Review.
-
Novel sensitive isothermal-based diagnostic technique for the detection of African swine fever virus.Arch Virol. 2023 Feb 5;168(3):79. doi: 10.1007/s00705-023-05702-z. Arch Virol. 2023. PMID: 36740635
-
Development of a TaqMan-Probe-Based Multiplex Real-Time PCR for the Simultaneous Detection of African Swine Fever Virus, Porcine Circovirus 2, and Pseudorabies Virus in East China from 2020 to 2022.Vet Sci. 2023 Feb 1;10(2):106. doi: 10.3390/vetsci10020106. Vet Sci. 2023. PMID: 36851410 Free PMC article.
-
African Swine Fever: Epidemiology, the Design of New Diagnostic Methods, and Vaccine Development.Pathogens. 2023 Aug 15;12(8):1042. doi: 10.3390/pathogens12081042. Pathogens. 2023. PMID: 37624002 Free PMC article.
References
Grants and funding
- grant no. 320071-02/The Animal Disease Management Technology Development Program of the Ministry of Agriculture, Food, and Rural Affairs, Republic of Korea.
- grant no. 122011-2/The Animal Disease Management Technology Advancement Support Program of the Ministry of Agriculture, Food, and Rural Affairs, Republic of Korea.
LinkOut - more resources
Full Text Sources

