Prognostic Markers of Ocrelizumab Effectiveness in Multiple Sclerosis: A Real World Observational Multicenter Study
- PMID: 35456175
- PMCID: PMC9029051
- DOI: 10.3390/jcm11082081
Prognostic Markers of Ocrelizumab Effectiveness in Multiple Sclerosis: A Real World Observational Multicenter Study
Abstract
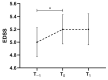
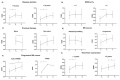
Pivotal trials showed the effectiveness of the monoclonal antibody ocrelizumab in relapsing and progressive multiple sclerosis (MS). However, data on everyday practice in MS patients and markers of treatment effectiveness are scarce. We aimed to collect real-world data from ocrelizumab-treated MS patients, relapsing-remitting (RR) and progressive MS patients (PMS), including active secondary progressive MS (aSPMS) and primary progressive MS (PPMS) patients, and to explore potential prognostic factors of clinical outcome. Patients were enrolled at MS centres in the Campania region, Italy. We collected clinic-demographic features retrospectively one year before ocrelizumab start (T−1), at ocrelizumab start (T0), and after one year from ocrelizumab start (T1). We explored possible clinical markers of treatment effectiveness in those patients receiving ocrelizumab treatment for at least one year using multilevel-mixed models. We included a total of 383 MS patients (89 RRMS and 294 PMS; 205 females, mean age: 45.8 ± 11.2, disease duration: 12.7 ± 11.6 years). Patients had a mean follow-up of 12.4 ± 8.2 months, and 217 patients completed one-year ocrelizumab treatment. Overall, EDSS increased from T−1 to T0 (coeff. = 0.30, 95% coefficient interval [CI] = 0.19−0.41, p < 0.001) without a further change between T0 and T1 (p = 0.61). RRMS patients did not show an EDSS change between T−1 and T0 nor between T0 and T1. Conversely, PMS patients showed EDSS increase from T−1 to T0 (coeff. = 0.34, 95% CI = 0.22−0.45, p < 0.001) without a further change between T0 and T1 (p = 0.21). PMS patients with a time from conversion shorter than 2 years showed increased EDSS from T−1 to T0 (coeff. = 0.63, 95% CI = 0.18−1.08, p = 0.006) without a further change between T0 and T1 (p = 0.94), whereas PMS patients with a time from conversion longer than 2 years showed increased EDSS from T0 to T1 (coeff. = 0.30, 95% CI = 0.11−0.49, p = 0.002). Naïve patients showed an EDSS decrease between T0 and T1 (coeff. = −0.30, 95% CI = −0.50−−0.09, p = 0.004). In conclusion, our study highlighted that early ocrelizumab treatment is effective in modifying the disability accrual in MS patients.
Keywords: disease-modifying treatment; multiple sclerosis; ocrelizumab; progression; real-world.
Conflict of interest statement
Antonio Carotenuto received research grants from ALMIRALL, and honoraria from Novartis, Merck, and Biogen. Roberta Lanzillo received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, and Almirall. Vincenzo Brescia Morra received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, and Almirall. Elisabetta Signoriello received speaker honoraria and/or consultancy fees from Biogen, Teva, Genzyme, Merck, Novartis, Almirall, and Roche. Marcello Moccia has received research grants from MAGNIMS-ECTRIMS, UK MS Society, and Merck; and honoraria from Ipsen, Merck, Roche, and Sanofi-Genzyme. Giacomo Lus received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, Almirall, Merz, and Ipsen. Simona Bonavita received speaker honoraria and/or advisory board from Biogen-Idec, Sanofi-Genzyme, Bristol-Meyers, Merck-Serono, Novartis and Viatris. Leonardo Sinisi received advisory board honoraria and congress grants from Almirall, Biogen, Sanofi-Genzyme, Bristol-Meyers, Merck, Novartis, Viatris. Maria Petracca discloses travel/meeting expenses from Novartis, Roche and Merck, speaking honoraria from HEALTH&LIFE S.r.l., honoraria for consulting services from Biogen, and research grants from the Italian MS Foundation. Antonio Gallo received personal compensation for speaking and consultancy from Biogen, Bristol Myers Squibb, Merck-Serono, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva. Maria Di Gregorio received grants for traveling and congress participation by Biogen, Merck, Novartis, Teva, Roche and Sanofi-Genzyme. She also served as an advisory board member for Biogen, Merck, Novartis, and Bristol Myers Squibb.
Figures
References
-
- Kappos L., Li D., Calabresi P.A., O’Connor P., Bar-Or A., Barkhof F., Yin M., Leppert D., Glanzman R., Tinbergen J., et al. Ocrelizumab in relapsing-remitting multiple sclerosis: A phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. doi: 10.1016/S0140-6736(11)61649-8. - DOI - PubMed
-
- Rojas J.I., Patrucco L., Fruns M., Hornung G., Flores J., Carnero Contentti E., Lopez P.A., Pettinicchi J.P., Caride A., Galleguillos L., et al. Real-world experience of ocrelizumab in multiple sclerosis patients in Latin America. Arq. Neuro-Psiquiatr. 2021;79:305–309. doi: 10.1590/0004-282x-anp-2020-0339. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

