Strategies for Facilitating Totally Percutaneous Transfemoral TAVR Procedures
- PMID: 35456197
- PMCID: PMC9028438
- DOI: 10.3390/jcm11082104
Strategies for Facilitating Totally Percutaneous Transfemoral TAVR Procedures
Abstract
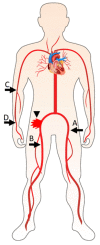
Transcatheter aortic valve replacement (TAVR) has transformed the treatment of aortic stenosis and should ideally be performed as a totally percutaneous procedure via the transfemoral (TF) approach. Peripheral vascular disease may impede valve delivery, and vascular access site complications are associated with adverse clinical outcome and increased mortality. We review strategies aimed to facilitate TF valve delivery in patients with hostile vascular anatomy and achieve percutaneous management of vascular complications.
Keywords: percutaneous; transcatheter aortic valve replacement; transfemoral.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Siontis G.C.M., Overtchouk P., Cahill T.J., Modine T., Prendergast B., Praz F., Pilgrim T., Petrinic T., Nikolakopoulou A., Salanti G., et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019;40:3143–3153. doi: 10.1093/eurheartj/ehz275. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

