Endoscopic Intragastric Injection of Botulinum Toxin A in Obese Patients Accelerates Weight Loss after Bariatric Surgery: Follow-Up of a Randomised Controlled Trial (IntraTox Study)
- PMID: 35456220
- PMCID: PMC9027998
- DOI: 10.3390/jcm11082126
Endoscopic Intragastric Injection of Botulinum Toxin A in Obese Patients Accelerates Weight Loss after Bariatric Surgery: Follow-Up of a Randomised Controlled Trial (IntraTox Study)
Abstract
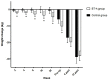
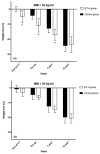
Background: Intragastric injection of botulinum toxin A (BT-A) has been shown to be effective for weight loss up to six months after administration, according to previous studies. Our objective was to determine, in patients on bariatric surgery waiting lists, the effect of BT-A on weight loss in the pre- and postoperative period and to analyse if there are different responses based on Body Mass Index (BMI). Methods: We performed a follow-up analysis of the IntraTox study, which included 46 patients on bariatric surgery waiting lists in a single-centre, randomised, double-blind, placebo-controlled clinical trial. The treatment group received intragastric BT-A, whereas the control group received physiological saline solution. The one-time procedure was performed at the time of diagnostic endoscopy 7−8 months before surgery. Weight loss was evaluated at admission and after 4 and 12 weeks from the bariatric surgery. Our analysis was stratified by BMI at randomisation. Results: weight loss percentage on the day of surgery, with respect to the initial visit, was −4.5 ± 3.9% for the control group vs. −7.6 ± 4.2%, for the treatment group (p = 0.013). Weight loss percentage tended to remain greater in the treatment group one month after the intervention (−12.7 ± 4.7% vs. −15.2 ± 4.6%, p = 0.07) and become similar three months after (−21.6 ± 4.7% vs. −21.6 ± 4.6%). After stratifying by BMI, only patients with BMI over 50 kg/m2 allocated to the treatment group obtained a greater weight loss at the end of the trial, the day of surgery, and one month after, compared with the placebo group (−4.9 ± 4.9%, −10.8 ± 5.3% and −17.1 ± 3.8% vs. −0.1 ± 2.6%, −4.3 ± 3.2% and −12.8 ± 4.1%, respectively (p < 0.05). Conclusions: intragastric injection of BT-A is effective to achieve significant weight loss, especially in extreme obesity. Its use before bariatric surgery enhances perioperative weight loss.
Keywords: bariatric surgery; botulinum toxin; endoscopy; obesity; weight loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y., Bahalim A.N., et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9•1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
-
- Sangiorgi G.M., Cereda A., Porchetta N., Benedetto D., Matteucci A., Bonanni M., Chiricolo G., De Lorenzo A. Endovascular Bariatric Surgery as Novel Minimally Invasive Technique for Weight Management in the Morbidly Obese: Review of the Literature. Nutrients. 2021;13:2541. doi: 10.3390/nu13082541. - DOI - PMC - PubMed
-
- Sánchez Torralvo F.J., Valdés Hernández S., Tapia M.J., Abuín Fernández J., Olveira G. Intragastric injection of botulinum toxin. A real alternative for obesity treatment? A systematic review. Nutr. Hosp. 2017;34:1482–1488. - PubMed

