Epigenetic Risks of Medically Assisted Reproduction
- PMID: 35456243
- PMCID: PMC9027760
- DOI: 10.3390/jcm11082151
Epigenetic Risks of Medically Assisted Reproduction
Abstract
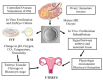
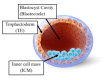
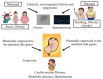
Since the birth of Louise Joy Brown, the first baby conceived via in vitro fertilization, more than 9 million children have been born worldwide using assisted reproductive technologies (ART). In vivo fertilization takes place in the maternal oviduct, where the unique physiological conditions guarantee the healthy development of the embryo. During early embryogenesis, a major wave of epigenetic reprogramming takes place that is crucial for the correct development of the embryo. Epigenetic reprogramming is susceptible to environmental changes and non-physiological conditions such as those applied during in vitro culture, including shift in pH and temperature, oxygen tension, controlled ovarian stimulation, intracytoplasmic sperm injection, as well as preimplantation embryo manipulations for genetic testing. In the last decade, concerns were raised of a possible link between ART and increased incidence of imprinting disorders, as well as epigenetic alterations in the germ cells of infertile parents that are transmitted to the offspring following ART. The aim of this review was to present evidence from the literature regarding epigenetic errors linked to assisted reproduction treatments and their consequences on the conceived children. Furthermore, we provide an overview of disease risk associated with epigenetic or imprinting alterations in children born via ART.
Keywords: assisted reproductive technology; epigenetics; human in vitro fertilization; imprinting disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- De Geyter C., Wyns C., Calhaz-Jorge C., De Mouzon J., Ferraretti A.P., Kupka M., Andersen A.N., Nygren K.G., Goossens V. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum. Reprod. 2020;35:2832–2849. doi: 10.1093/humrep/deaa250. - DOI - PMC - PubMed
-
- Thoma M.E., McLain A., Louis J.F., King R.B., Trumble A.C., Sundaram R., Louis G.B. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013;99:1324–1331. doi: 10.1016/j.fertnstert.2012.11.037. - DOI - PMC - PubMed
-
- Ventura-Juncá P., Irarrázaval I., Rolle A.J., Gutiérrez J.I., Moreno R.D., Santos M.J. In vitro fertilization (IVF) in mammals: Epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 2015;48:68. doi: 10.1186/s40659-015-0059-y. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

