Development of CAR T Cell Therapy in Children-A Comprehensive Overview
- PMID: 35456250
- PMCID: PMC9024694
- DOI: 10.3390/jcm11082158
Development of CAR T Cell Therapy in Children-A Comprehensive Overview
Abstract
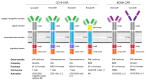
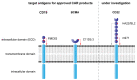
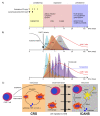
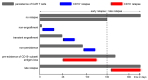
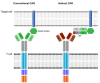
CAR T cell therapy has revolutionized immunotherapy in the last decade with the successful establishment of chimeric antigen receptor (CAR)-expressing cellular therapies as an alternative treatment in relapsed and refractory CD19-positive leukemias and lymphomas. There are fundamental reasons why CAR T cell therapy has been approved by the Food and Drug administration and the European Medicines Agency for pediatric and young adult patients first. Commonly, novel therapies are developed for adult patients and then adapted for pediatric use, due to regulatory and commercial reasons. Both strategic and biological factors have supported the success of CAR T cell therapy in children. Since there is an urgent need for more potent and specific therapies in childhood malignancies, efforts should also include the development of CAR therapeutics and expand applicability by introducing new technologies. Basic aspects, the evolution and the drawbacks of childhood CAR T cell therapy are discussed as along with the latest clinically relevant information.
Keywords: FDA-approved CAR products; TcR versus CAR; evolution of CAR T cells; future directions of CAR T cell therapy; limitations and complications of CAR T cell therapy.
Conflict of interest statement
M.B., A.J., Z.L. and S.F.Y. declare no conflict of interest. P.S. is coinventor of a patent application focusing on adapter CAR technology.
Figures


References
-
- Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

