Melittin from Bee Venom Encapsulating Electrospun Fibers as a Potential Antimicrobial Wound Dressing Patches for Skin Infections
- PMID: 35456558
- PMCID: PMC9030956
- DOI: 10.3390/pharmaceutics14040725
Melittin from Bee Venom Encapsulating Electrospun Fibers as a Potential Antimicrobial Wound Dressing Patches for Skin Infections
Abstract
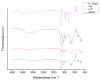
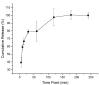
Skin infection compromises the body's natural defenses. Several antibiotics are no longer effective owing to the evolution of antimicrobial-resistant (AMR) bacteria, hence, the constant development of novel antibacterial agents. Naturally occurring antibacterial agents may be potential candidates for AMR bacterial infection treatments; however, caution should be taken when administering such agents due to the high incidence of toxicity. A fibrous material system from a biocompatible polymer that could be used as a skin patch for skin infections treatment caused by AMR bacteria is proposed in this study. Bee venom's active ingredient, melittin, was fabricated using electrospinning technology. Scanning electron microscopy showed that melittin-loaded fibers had smooth surfaces with no signs of beads or pores. The average diameter of this fibrous system was measured to be 1030 ± 160 nm, indicating its successful preparation. The melittin fibers' drug loading and entrapment efficiency (EE%) were 49 ± 3 µg/mg and 84 ± 5%, respectively. This high EE% can be another successful preparatory criterion. An in vitro release study demonstrated that 40% of melittin was released after 5 min and achieved complete release after 120 min owing to the hydrophilic nature of the PVP polymer. A concentration of ≤10 µg/mL was shown to be safe for use on human dermal fibroblasts HFF-1 after 24-h exposure, while an antibacterial MIC study found that 5 μg/mL was the effective antimicrobial concentration for S. aureus, A. baumannii, E. coli and Candida albicans yeast. A melittin-loaded fibrous system demonstrated an antibacterial zone of inhibition equivalent to the control (melittin discs), suggesting its potential use as a wound dressing patch for skin infections.
Keywords: antimicrobial resistant bacteria; antimicrobial wound dressing; bee venom; electrospinning; electrospun fibers; melittin; skin infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

