Inorganic Nanoparticles in Bone Healing Applications
- PMID: 35456604
- PMCID: PMC9027776
- DOI: 10.3390/pharmaceutics14040770
Inorganic Nanoparticles in Bone Healing Applications
Abstract
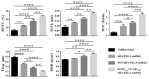
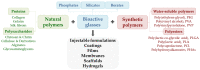
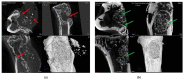
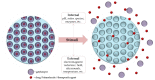
Modern biomedicine aims to develop integrated solutions that use medical, biotechnological, materials science, and engineering concepts to create functional alternatives for the specific, selective, and accurate management of medical conditions. In the particular case of tissue engineering, designing a model that simulates all tissue qualities and fulfills all tissue requirements is a continuous challenge in the field of bone regeneration. The therapeutic protocols used for bone healing applications are limited by the hierarchical nature and extensive vascularization of osseous tissue, especially in large bone lesions. In this regard, nanotechnology paves the way for a new era in bone treatment, repair and regeneration, by enabling the fabrication of complex nanostructures that are similar to those found in the natural bone and which exhibit multifunctional bioactivity. This review aims to lay out the tremendous outcomes of using inorganic nanoparticles in bone healing applications, including bone repair and regeneration, and modern therapeutic strategies for bone-related pathologies.
Keywords: bioceramic nanoparticles; bone regeneration; inorganic nanoparticles; metallic nanoparticles; oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ralston S.H. Bone structure and metabolism. Medicine. 2021;49:567–571. doi: 10.1016/j.mpmed.2021.06.009. - DOI
-
- Abe K., Shimozaki S., Domoto T., Yamamoto N., Tsuchiya H., Minamoto T. Glycogen synthase kinase 3β biology in bone and soft tissue sarcomas. J. Cancer Metastasis Treat. 2020;6:51. doi: 10.20517/2394-4722.2020.117. - DOI
Publication types
LinkOut - more resources
Full Text Sources

