Regulation of p27 (Kip1) by Ubiquitin E3 Ligase RNF6
- PMID: 35456636
- PMCID: PMC9029106
- DOI: 10.3390/pharmaceutics14040802
Regulation of p27 (Kip1) by Ubiquitin E3 Ligase RNF6
Abstract
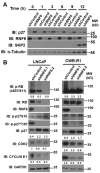
The cyclin-dependent kinase inhibitor p27 (Kip1) is an important regulator of the G1/S checkpoint. It is degraded by the SCF-SKP2 complex in late G1 thereby allowing cells to progress to the S phase. Here we investigated the role of the E3 ubiquitin ligase RNF6 (Ring Finger Protein 6) in cell cycle progression in prostate cancer cells. Our data demonstrate that RNF6 can promote cell cycle progression by reducing the levels of p27. Knockdown of RNF6 led to an increase in the stability of p27 and to the arrest of cells in the G1 phase. RNF6 interacted with p27 via its KIL domain and this interaction was found to be phosphorylation independent. RNF6 enhanced ubiquitination and subsequent degradation of p27 in the early G0/G1 phase of the cell cycle. Knockdown of RNF6 expression by short hairpin RNA led to inhibition of the CDK2/Cyclin E complex thereby reducing phosphorylation of Retinoblastoma protein (Rb) and to a subsequent decrease in cell cycle progression and proliferation. Our data suggest that RNF6 acts as a negative regulator for p27kip1 leading to its proteasome-dependent degradation in the early G0/G1 phase of the cell cycle.
Keywords: RNF6; cell cycle; p27; prostate cancer.
Conflict of interest statement
The author (Jin Xu) is currently an employee of AstraZeneca, however, he did not work for AstraZeneca at the time of experiments and manuscript preparation. The other authors declare no conflict of interest.
Figures





References
-
- Jiao B., Taniguchi-Ishigaki N., Güngör C., Peters M.A., Chen Y.-W., Riethdorf S., Drung A., Ahronian L.G., Shin J., Pagnis R., et al. Functional activity of RLIM/Rnf12 is regulated by phosphorylation-dependent nucleocytoplasmic shuttling. Mol. Biol. Cell. 2013;24:3085–3096. doi: 10.1091/mbc.e13-05-0239. - DOI - PMC - PubMed
-
- Tursun B., Schlüter A., Peters M.A., Viehweger B., Ostendorff H.P., Soosairajah J., Drung A., Bossenz M., Johnsen S.A., Schweizer M., et al. The ubiquitin ligase Rnf6 regulates local LIM kinase 1 levels in axonal growth cones. Genes Dev. 2005;19:2307–2319. doi: 10.1101/gad.1340605. - DOI - PMC - PubMed
-
- Takahashi H., Uematsu A., Yamanaka S., Imamura M., Nakajima T., Doi K., Yasuoka S., Takahashi C., Takeda H., Sawasaki T. Establishment of a Wheat Cell-Free Synthesized Protein Array Containing 250 Human and Mouse E3 Ubiquitin Ligases to Identify Novel Interaction between E3 Ligases and Substrate Proteins. PLoS ONE. 2016;11:e0156718. doi: 10.1371/journal.pone.0156718. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

