Non-Effective Improvement of Absorption for Some Nanoparticle Formulations Explained by Permeability under Non-Sink Conditions
- PMID: 35456650
- PMCID: PMC9024805
- DOI: 10.3390/pharmaceutics14040816
Non-Effective Improvement of Absorption for Some Nanoparticle Formulations Explained by Permeability under Non-Sink Conditions
Abstract
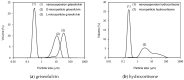
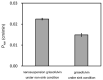
We evaluated the in vitro permeability of nanoparticle formulations of high and low lipophilic compounds under non-sink conditions, wherein compounds are not completely dissolved. The permeability of the highly lipophilic compound, griseofulvin, was improved by about 30% due to nanonization under non-sink conditions. Moreover, this permeability was about 50% higher than that under sink conditions. On the other hand, for the low lipophilic compound, hydrocortisone, there was no difference in permeability between micro-and nano-sized compounds under non-sink conditions. The nanonization of highly lipophilic compounds improves the permeability of the unstirred water layer (UWL), which in turn improves overall permeability. On the other hand, because the rate-limiting step in permeation for the low lipophilic compounds is the diffusion of the compounds in the membrane, the improvement of UWL permeability by nanonization does not improve the overall permeability. Based on this mechanism, nanoparticle formulations are not effective for low lipophilic compounds. To accurately predict the absorption of nanoparticle formulations, it is necessary to consider their permeability under non-sink conditions which reflect in vivo conditions.
Keywords: absorption; lipophilicity; nanoparticle formulation; non-sink condition; permeability.
Conflict of interest statement
K.S. and N.T. are the researchers currently employed by Chugai Pharmaceutical Co., Ltd. The funders/companies had no role: in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no conflict of interest.
Figures










References
-
- Abrahamsson B., McAllister M., Augustijns P., Zane P., Butler J., Holm R., Langguth P., Lindahl A., Müllertz A., Pepin X., et al. Six years of progress in the oral biopharmaceutics area—A summary from the IMI OrBiTo project. Eur. J. Pharm. Biopharm. 2020;152:236–247. doi: 10.1016/j.ejpb.2020.05.008. - DOI - PubMed
LinkOut - more resources
Full Text Sources

