Fluid-Phase Endocytosis and Lysosomal Degradation of Bovine Lactoferrin in Lung Cells
- PMID: 35456688
- PMCID: PMC9032238
- DOI: 10.3390/pharmaceutics14040855
Fluid-Phase Endocytosis and Lysosomal Degradation of Bovine Lactoferrin in Lung Cells
Abstract
The iron-binding protein lactoferrin and the cell-penetrating peptides derived from its sequence utilise endocytosis to enter different cell types. The full-length protein has been extensively investigated as a potential therapeutic against a range of pathogenic bacteria, fungi, and viruses, including SARS-CoV-2. As a respiratory antiviral agent, several activity mechanisms have been demonstrated for lactoferrin, at the extracellular and plasma membrane levels, but as a protein that enters cells it may also have intracellular antiviral activity. Characterisation of lactoferrin's binding, endocytic traffic to lysosomes, or recycling endosomes for exocytosis is lacking, especially in lung cell models. Here, we use confocal microscopy, flow cytometry, and degradation assays to evaluate binding, internalisation, endocytic trafficking, and the intracellular fate of bovine lactoferrin in human lung A549 cells. In comparative studies with endocytic probes transferrin and dextran, we show that lactoferrin binds to negative charges on the cell surface and actively enters cells via fluid-phase endocytosis, in a receptor-independent manner. Once inside the cell, we show that it is trafficked to lysosomes where it undergoes degradation within two hours. These findings provide opportunities for investigating both lactoferrin and derived cell-penetrating peptides activities of targeting intracellular pathogens.
Keywords: endocytosis; intracellular trafficking; lactoferrin; lysosomal degradation.
Conflict of interest statement
E.J.S., I.P., P.W. and A.T.J. declare no conflict of interest. P.H. is founder of Virustatic Limited, and L.H. is an employee of Virustatic Limited; both declare no conflict of interest. The funders and company had no role in the collection, analyses, and interpretation of data.
Figures
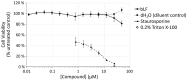
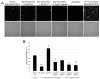
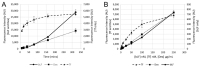
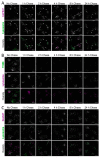
References
-
- Duchardt F., Ruttekolk I.R., Verdurmen W.P.R., Lortat-Jacob H., Burck J., Hufnagel H., Fischer R., van den Heuvel M., Lowik D., Vuister G.W., et al. A cell-penetrating peptide derived from human lactoferrin with conformation-dependent uptake efficiency. J. Biol. Chem. 2009;284:36099–36108. doi: 10.1074/jbc.M109.036426. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

