Multilayer Regulation of Neisseria meningitidis NHBA at Physiologically Relevant Temperatures
- PMID: 35456883
- PMCID: PMC9031163
- DOI: 10.3390/microorganisms10040834
Multilayer Regulation of Neisseria meningitidis NHBA at Physiologically Relevant Temperatures
Abstract
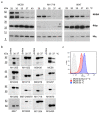
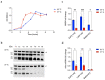
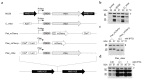
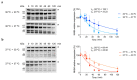
Neisseria meningitidis colonizes the nasopharynx of humans, and pathogenic strains can disseminate into the bloodstream, causing septicemia and meningitis. NHBA is a surface-exposed lipoprotein expressed by all N. meningitidis strains in different isoforms. Diverse roles have been reported for NHBA in heparin-mediated serum resistance, biofilm formation, and adherence to host tissues. We determined that temperature controls the expression of NHBA in all strains tested, with increased levels at 30−32 °C compared to 37 °C. Higher NHBA expression at lower temperatures was measurable both at mRNA and protein levels, resulting in higher surface exposure. Detailed molecular analysis indicated that multiple molecular mechanisms are responsible for the thermoregulated NHBA expression. The comparison of mRNA steady-state levels and half-lives at 30 °C and 37 °C demonstrated an increased mRNA stability/translatability at lower temperatures. Protein stability was also impacted, resulting in higher NHBA stability at lower temperatures. Ultimately, increased NHBA expression resulted in higher susceptibility to complement-mediated killing. We propose that NHBA regulation in response to temperature downshift might be physiologically relevant during transmission and the initial step(s) of interaction within the host nasopharynx. Together these data describe the importance of NHBA both as a virulence factor and as a vaccine antigen during neisserial colonization and invasion.
Keywords: 4CmenB; NHBA; Neisseria meningitidis; thermoregulation; vaccine.
Conflict of interest statement
At the time of the study S.B. and T.B. were recipients of a GSK fellowship from the PhD program of the University of Bologna. M.S. and I.D. are employees of the GSK group of companies; A.A. and A.F.H. were employees of Novartis Vaccines & Diagnostics (NVD) Srl or, later, GSK at the time of the study. E.N. was a recipient of a GSK fellowship from the PhD program of the University of Siena. I.D. reports ownership of GSK stocks. I.D. is listed as inventor on patents on vaccine candidates owned by the GSK group of companies. V.S. declares no conflicts of interest. The funder GlaxoSmithKline Biologicals SA had a role in the study design, collection, analysis, interpretation of data and the writing of the manuscript as well as the decision to submit for its publication.
Figures

References
LinkOut - more resources
Full Text Sources

