Emerging Antiarrhythmic Drugs for Atrial Fibrillation
- PMID: 35456912
- PMCID: PMC9029767
- DOI: 10.3390/ijms23084096
Emerging Antiarrhythmic Drugs for Atrial Fibrillation
Abstract
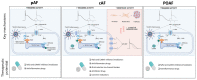
Atrial fibrillation (AF), the most common cardiac arrhythmia worldwide, is driven by complex mechanisms that differ between subgroups of patients. This complexity is apparent from the different forms in which AF presents itself (post-operative, paroxysmal and persistent), each with heterogeneous patterns and variable progression. Our current understanding of the mechanisms responsible for initiation, maintenance and progression of the different forms of AF has increased significantly in recent years. Nevertheless, antiarrhythmic drugs for the management of AF have not been developed based on the underlying arrhythmia mechanisms and none of the currently used drugs were specifically developed to target AF. With the increased knowledge on the mechanisms underlying different forms of AF, new opportunities for developing more effective and safer AF therapies are emerging. In this review, we provide an overview of potential novel antiarrhythmic approaches based on the underlying mechanisms of AF, focusing both on the development of novel antiarrhythmic agents and on the possibility of repurposing already marketed drugs. In addition, we discuss the opportunity of targeting some of the key players involved in the underlying AF mechanisms, such as ryanodine receptor type-2 (RyR2) channels and atrial-selective K+-currents (IK2P and ISK) for antiarrhythmic therapy. In addition, we highlight the opportunities for targeting components of inflammatory signaling (e.g., the NLRP3-inflammasome) and upstream mechanisms targeting fibroblast function to prevent structural remodeling and progression of AF. Finally, we critically appraise emerging antiarrhythmic drug principles and future directions for antiarrhythmic drug development, as well as their potential for improving AF management.
Keywords: atrial fibrillation; ectopic activity; fibroblast; pharmacology.
Conflict of interest statement
Dobromir Dobrev is member of the scientific advisory boards of Acesion Pharma and Omeicos Therapeutics GmbH. The other authors have nothing to disclose.
Figures
References
-
- Wang T.J., Larson M.G., Levy D., Vasan R.S., Leip E.P., Wolf P.A., D’Agostino R.B., Murabito J.M., Kannel W.B., Benjamin E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

