Opioidergic Signaling-A Neglected, Yet Potentially Important Player in Atopic Dermatitis
- PMID: 35456955
- PMCID: PMC9027603
- DOI: 10.3390/ijms23084140
Opioidergic Signaling-A Neglected, Yet Potentially Important Player in Atopic Dermatitis
Abstract
Atopic dermatitis (AD) is one of the most common skin diseases, the prevalence of which is especially high among children. Although our understanding about its pathogenesis has substantially grown in recent years, and hence, several novel therapeutic targets have been successfully exploited in the management of the disease, we still lack curative treatments for it. Thus, there is an unmet societal demand to identify further details of its pathogenesis to thereby pave the way for novel therapeutic approaches with favorable side effect profiles. It is commonly accepted that dysfunction of the complex cutaneous barrier plays a central role in the development of AD; therefore, the signaling pathways involved in the regulation of this quite complex process are likely to be involved in the pathogenesis of the disease and can provide novel, promising, yet unexplored therapeutic targets. Thus, in the current review, we aim to summarize the available potentially AD-relevant data regarding one such signaling pathway, namely cutaneous opioidergic signaling.
Keywords: atopic dermatitis (AD); cutaneous barrier; inflammation; itch; keratinocyte; mast cell; nociceptin/orphanin FQ (NOP) receptor; opioid; skin; µ-opioid receptor (MOR); δ-opioid receptor (DOR); κ-opioid receptor (KOR).
Conflict of interest statement
A.O. provides consultancy services to Monasterium Laboratory Skin & Hair Research Solutions GmbH. Neither the said company, nor the above funding sponsors had a role in the writing of the manuscript or in the decision to publish it. Thus, the authors declare no conflict of interest.
Figures
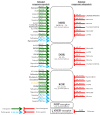


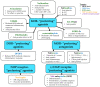
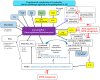
References
Publication types
MeSH terms
Substances
Grants and funding
- 134235/National Research, Development and Innovation Office
- 134725/National Research, Development and Innovation Office
- 134993/National Research, Development and Innovation Office
- GINOP-2.3.2-15-2016-00026/National Research, Development and Innovation Office
- EFOP-3.6.3-VEKOP-16-2017-00009/National Research, Development and Innovation Office
LinkOut - more resources
Full Text Sources
Research Materials

