Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors
- PMID: 35456962
- PMCID: PMC9032593
- DOI: 10.3390/ijms23084144
Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors
Abstract
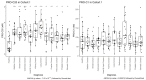
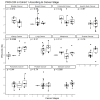
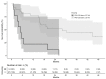
In the tumor microenvironment, the extracellular matrix (ECM) has been recognized as an important part of cancer development. The dominant ECM proteins are the 28 types of collagens, each with a unique function in tissue architecture. Type XX collagen, however, is poorly characterized, and little is known about its involvement in cancer. We developed an ELISA quantifying type XX collagen, named PRO-C20, using a monoclonal antibody raised against the C-terminus. PRO-C20 and PRO-C1, an ELISA targeting the N-terminal pro-peptide of type I collagen, was measured in sera of 219 patients with various solid cancer types and compared to sera levels of 33 healthy controls. PRO-C20 was subsequently measured in a separate cohort comprising 36 patients with pancreatic ductal adenocarcinoma (PDAC) and compared to 20 healthy controls and 11 patients with chronic pancreatitis. PRO-C20 was significantly elevated in all cancers tested: bladder, breast, colorectal, head and neck, kidney, lung, melanoma, ovarian, pancreatic, prostate, and stomach cancer (p < 0.01−p < 0.0001). PRO-C1 was only elevated in patients with ovarian cancer. PRO-C20 could discriminate between patients and healthy controls with AUROC values ranging from 0.76 to 0.92. Elevated levels were confirmed in a separate cohort of patients with PDAC (p < 0.0001). High PRO-C20 levels (above 2.57 nM) were predictive of poor survival after adjusting for the presence of metastasis, age, and sex (HR: 4.25, 95% CI: 1.52−11.9, p-value: 0.006). Circulating type XX collagen is elevated in sera of patients with various types of cancer and has prognostic value in PDAC. If validated, PRO-C20 may be a novel biomarker for patients with solid tumors and can help understand the ECM biology of cancer.
Keywords: ECM; PDAC; biomarker; cancer; serum; type XX collagen.
Conflict of interest statement
A patent has been filed for the PRO-C20 ELISA. JTU, CJ, EAM, NIN, TMJ, MK, and NW are employed at Nordic Bioscience A/S, a biotech company involved in the development of biomarkers. MK and NW own stock in Nordic Bioscience A/S.
Figures



Similar articles
-
Type XXII Collagen Complements Fibrillar Collagens in the Serological Assessment of Tumor Fibrosis and the Outcome in Pancreatic Cancer.Cells. 2022 Nov 24;11(23):3763. doi: 10.3390/cells11233763. Cells. 2022. PMID: 36497023 Free PMC article.
-
Levels of type XVII collagen (BP180) ectodomain are elevated in circulation from patients with multiple cancer types and is prognostic for patients with metastatic colorectal cancer.BMC Cancer. 2023 Oct 6;23(1):949. doi: 10.1186/s12885-023-11470-5. BMC Cancer. 2023. PMID: 37803411 Free PMC article.
-
Noninvasive prognostic biomarker potential of quantifying the propeptides of Type XI collagen alpha-1 chain (PRO-C11) in patients with pancreatic ductal adenocarcinoma.Int J Cancer. 2021 Jul 1;149(1):228-238. doi: 10.1002/ijc.33551. Epub 2021 Mar 22. Int J Cancer. 2021. PMID: 33687786
-
Targeting the tumor microenvironment for pancreatic ductal adenocarcinoma therapy.Chin Clin Oncol. 2019 Apr;8(2):18. doi: 10.21037/cco.2019.03.02. Chin Clin Oncol. 2019. PMID: 31070038 Review.
-
Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma.Int J Cancer. 2013 Mar 1;132(5):993-1003. doi: 10.1002/ijc.27715. Epub 2012 Oct 5. Int J Cancer. 2013. PMID: 22777597 Review.
Cited by
-
Assessing Breast Cancer through Tumor Microenvironment Mapping of Collagen and Other Biomolecule Spectral Fingerprints─A Review.ACS Sens. 2024 Sep 27;9(9):4364-4379. doi: 10.1021/acssensors.4c00585. Epub 2024 Aug 22. ACS Sens. 2024. PMID: 39175278 Free PMC article. Review.
-
Collagen in pituitary adenomas: A comprehensive review of biological roles and clinical implications.J Clin Transl Endocrinol. 2025 Jul 10;41:100408. doi: 10.1016/j.jcte.2025.100408. eCollection 2025 Sep. J Clin Transl Endocrinol. 2025. PMID: 40697902 Free PMC article. Review.
-
Type XXII Collagen Complements Fibrillar Collagens in the Serological Assessment of Tumor Fibrosis and the Outcome in Pancreatic Cancer.Cells. 2022 Nov 24;11(23):3763. doi: 10.3390/cells11233763. Cells. 2022. PMID: 36497023 Free PMC article.
-
The Multifaced Role of Collagen in Cancer Development and Progression.Int J Mol Sci. 2024 Dec 17;25(24):13523. doi: 10.3390/ijms252413523. Int J Mol Sci. 2024. PMID: 39769286 Free PMC article. Review.
-
Tumor-associated fibrosis: a unique mechanism promoting ovarian cancer metastasis and peritoneal dissemination.Cancer Metastasis Rev. 2024 Sep;43(3):1037-1053. doi: 10.1007/s10555-024-10169-8. Epub 2024 Mar 28. Cancer Metastasis Rev. 2024. PMID: 38546906 Free PMC article. Review.
References
-
- Alix-Panabières C., Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

