Investigating Biomarkers for USH2A Retinopathy Using Multimodal Retinal Imaging
- PMID: 35457016
- PMCID: PMC9024786
- DOI: 10.3390/ijms23084198
Investigating Biomarkers for USH2A Retinopathy Using Multimodal Retinal Imaging
Abstract
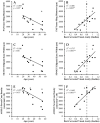
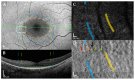
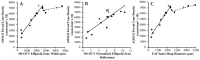
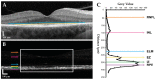
Pathogenic mutations in USH2A are a leading cause of visual loss secondary to non-syndromic or Usher syndrome-associated retinitis pigmentosa (RP). With an increasing number of RP-targeted clinical trials in progress, we sought to evaluate the photoreceptor topography underlying patterns of loss observed on clinical retinal imaging to guide surrogate endpoint selection in USH2A retinopathy. In this prospective cross-sectional study, twenty-five patients with molecularly confirmed USH2A-RP underwent fundus autofluorescence (FAF), spectral-domain optical coherence tomography (SD-OCT) and adaptive optics scanning laser ophthalmoscopy (AOSLO) retinal imaging. Analysis comprised measurement of FAF horizontal inner (IR) and outer (OR) hyperautofluorescent ring diameter; SD-OCT ellipsoid zone (EZ) and external limiting membrane (ELM) width, normalised EZ reflectance; AOSLO foveal cone density and intact macular photoreceptor mosaic (IMPM) diameter. Thirty-two eyes from 16 patients (mean age ± SD, 36.0 ± 14.2 years) with USH2A-associated Usher syndrome type 2 (n = 14) or non-syndromic RP (n = 2) met the inclusion criteria. Spatial alignment was observed between IR-EZ and OR-ELM diameters/widths (p < 0.001). The IMPM border occurred just lateral to EZ loss (p < 0.001), although sparser intact photoreceptor inner segments were detected until ELM disruption. EZ width and IR diameter displayed a biphasic relationship with cone density whereby slow cone loss occurred until retinal degeneration reached ~1350 μm from the fovea, beyond which greater reduction in cone density followed. Normalised EZ reflectance and cone density were significantly associated (p < 0.001). As the strongest correlate of cone density (p < 0.001) and best-corrected visual acuity (p < 0.001), EZ width is the most sensitive biomarker of structural and functional decline in USH2A retinopathy, rendering it a promising trial endpoint.
Keywords: USH2A; Usher syndrome; adaptive optics scanning laser ophthalmoscopy; fundus autofluorescence; retinitis pigmentosa; spectral-domain optical coherence tomography.
Conflict of interest statement
A.M.D. has received consulting fees from Boston Micromachines Corporation. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare no conflict of interest.
Figures


References
-
- Usher C. On the inheritance of retinitis pigmentosa, with notes of cases. R. Lond. Ophthalmol. Hosp. Rep. 1914;19:130–236.
-
- Kimberling W.J., Hildebrand M.S., Shearer A.E., Jensen M.L., Halder J.A., Trzupek K., Cohn E.S., Weleber R.G., Stone E.M., Smith R.J. Frequency of Usher syndrome in two pediatric populations: Implications for genetic screening of deaf and hard of hearing children. Genet. Med. 2010;12:512–516. doi: 10.1097/GIM.0b013e3181e5afb8. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

