Connexin Mutations and Hereditary Diseases
- PMID: 35457072
- PMCID: PMC9027513
- DOI: 10.3390/ijms23084255
Connexin Mutations and Hereditary Diseases
Abstract
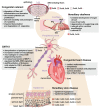
Inherited diseases caused by connexin mutations are found in multiple organs and include hereditary deafness, congenital cataract, congenital heart diseases, hereditary skin diseases, and X-linked Charcot-Marie-Tooth disease (CMT1X). A large number of knockout and knock-in animal models have been used to study the pathology and pathogenesis of diseases of different organs. Because the structures of different connexins are highly homologous and the functions of gap junctions formed by these connexins are similar, connexin-related hereditary diseases may share the same pathogenic mechanism. Here, we analyze the similarities and differences of the pathology and pathogenesis in animal models and find that connexin mutations in gap junction genes expressed in the ear, eye, heart, skin, and peripheral nerves can affect cellular proliferation and differentiation of corresponding organs. Additionally, some dominant mutations (e.g., Cx43 p.Gly60Ser, Cx32 p.Arg75Trp, Cx32 p.Asn175Asp, and Cx32 p.Arg142Trp) are identified as gain-of-function variants in vivo, which may play a vital role in the onset of dominant inherited diseases. Specifically, patients with these dominant mutations receive no benefits from gene therapy. Finally, the complete loss of gap junctional function or altered channel function including permeability (ions, adenosine triphosphate (ATP), Inositol 1,4,5-trisphosphate (IP3), Ca2+, glucose, miRNA) and electric activity are also identified in vivo or in vitro.
Keywords: congenital cataract; congenital heart diseases; connexin; gap junction; gene mutation; hereditary deafness; hereditary skin diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

