Neuroepigenetic Mechanisms of Action of Ultrashort Peptides in Alzheimer's Disease
- PMID: 35457077
- PMCID: PMC9032300
- DOI: 10.3390/ijms23084259
Neuroepigenetic Mechanisms of Action of Ultrashort Peptides in Alzheimer's Disease
Abstract
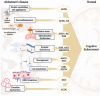
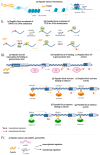
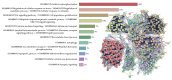
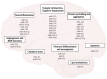
Epigenetic regulation of gene expression is necessary for maintaining higher-order cognitive functions (learning and memory). The current understanding of the role of epigenetics in the mechanism of Alzheimer's disease (AD) is focused on DNA methylation, chromatin remodeling, histone modifications, and regulation of non-coding RNAs. The pathogenetic links of this disease are the misfolding and aggregation of tau protein and amyloid peptides, mitochondrial dysfunction, oxidative stress, impaired energy metabolism, destruction of the blood-brain barrier, and neuroinflammation, all of which lead to impaired synaptic plasticity and memory loss. Ultrashort peptides are promising neuroprotective compounds with a broad spectrum of activity and without reported side effects. The main aim of this review is to analyze the possible epigenetic mechanisms of the neuroprotective action of ultrashort peptides in AD. The review highlights the role of short peptides in the AD pathophysiology. We formulate the hypothesis that peptide regulation of gene expression can be mediated by the interaction of short peptides with histone proteins, cis- and transregulatory DNA elements and effector molecules (DNA/RNA-binding proteins and non-coding RNA). The development of therapeutic agents based on ultrashort peptides may offer a promising addition to the multifunctional treatment of AD.
Keywords: Alzheimer’s disease; DNA; DNA-binding proteins; histones; neuroepigenetic; non-coding RNA; nucleosome; promotors; transcription factors; ultrashort peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

