A Novel NOX Inhibitor Treatment Attenuates Parkinson's Disease-Related Pathology in Mouse Models
- PMID: 35457082
- PMCID: PMC9030373
- DOI: 10.3390/ijms23084262
A Novel NOX Inhibitor Treatment Attenuates Parkinson's Disease-Related Pathology in Mouse Models
Abstract
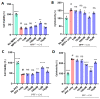
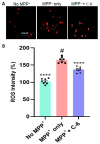
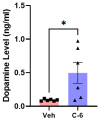
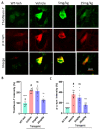
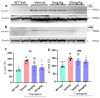
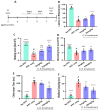
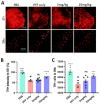
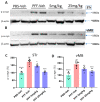
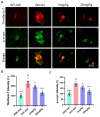
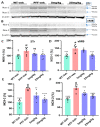
Parkinson's disease (PD) is a progressive neurodegenerative motor disorder without an available therapeutic to halt the formation of Lewy bodies for preventing dopaminergic neuronal loss in the nigrostriatal pathway. Since oxidative-stress-mediated damage has been commonly reported as one of the main pathological mechanisms in PD, we assessed the efficacy of a novel NOX inhibitor from AptaBio Therapeutics (C-6) in dopaminergic cells and PD mouse models. The compound reduced the cytotoxicity and enhanced the cell viability at various concentrations against MPP+ and α-synuclein preformed fibrils (PFFs). Further, the levels of ROS and protein aggregation were significantly reduced at the optimal concentration (1 µM). Using two different mouse models, we gavaged C-6 at two different doses to the PD sign-displaying transgenic mice for 2 weeks and stereotaxically PFF-injected mice for 5 weeks. Our results demonstrated that both C-6-treated mouse models showed alleviated motor deficits in pole test, hindlimb clasping, crossbeam, rotarod, grooming, and nesting analyses. We also confirmed that the compound treatment reduced the levels of protein aggregation, along with phosphorylated-α-synuclein, in the striatum and ventral midbrain and further dopaminergic neuronal loss. Taken together, our results strongly suggest that NOX inhibition can be a potential therapeutic target for PD.
Keywords: ROS inhibition; Thioflavin T; oxidative stress; protein aggregation; α-synuclein preformed fibrils.
Conflict of interest statement
This study was performed in collaboration between the Kim Lab at Delaware State University and AptaBio Therapeutics Inc. The novel compound was designed and synthesized by AptaBio Therapeutics, which partially supported this collaborative project. However, AptaBio Inc had no role in the experimental designs, data collection, analyses, and interpretation, or the writing of the manuscript for publication.
Figures


References
-
- Braak H., del Tredici K. Neuroanatomy and Pathology of Sporadic Parkinson’s Disease. Adv. Anat. Embryol. Cell Biol. 2009;201:1–119. - PubMed
-
- Stewart T., Sossi V., Aasly J.O., Wszolek Z.K., Uitti R.J., Hasegawa K., Yokoyama T., Zabetian C.P., Leverenz J.B., Stoessl A.J., et al. Phosphorylated α-synuclein in Parkinson’s disease: Correlation depends on disease severity. Acta Neuropathol. Commun. 2015;3:7. doi: 10.1186/s40478-015-0185-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

