Contribution of Whole-Genome Sequencing and Transcript Analysis to Decipher Retinal Diseases Associated with MFSD8 Variants
- PMID: 35457110
- PMCID: PMC9032189
- DOI: 10.3390/ijms23084294
Contribution of Whole-Genome Sequencing and Transcript Analysis to Decipher Retinal Diseases Associated with MFSD8 Variants
Abstract
Biallelic gene defects in MFSD8 are not only a cause of the late-infantile form of neuronal ceroid lipofuscinosis, but also of rare isolated retinal degeneration. We report clinical and genetic data of seven patients compound heterozygous or homozygous for variants in MFSD8, issued from a French cohort with inherited retinal degeneration, and two additional patients retrieved from a Swiss cohort. Next-generation sequencing of large panels combined with whole-genome sequencing allowed for the identification of twelve variants from which seven were novel. Among them were one deep intronic variant c.998+1669A>G, one large deletion encompassing exon 9 and 10, and a silent change c.750A>G. Transcript analysis performed on patients’ lymphoblastoid cell lines revealed the creation of a donor splice site by c.998+1669A>G, resulting in a 140 bp pseudoexon insertion in intron 10. Variant c.750A>G produced exon 8 skipping. In silico and in cellulo studies of these variants allowed us to assign the pathogenic effect, and showed that the combination of at least one severe variant with a moderate one leads to isolated retinal dystrophy, whereas the combination in trans of two severe variants is responsible for early onset severe retinal dystrophy in the context of late-infantile neuronal ceroid lipofuscinosis.
Keywords: MFSD8 gene; deep intronic variant; isolated macular dystrophy; neuronal ceroid lipofuscinosis; transcript analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
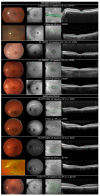
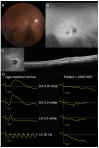
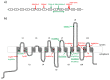

References
-
- Georgiou M., Kane T., Tanna P., Bouzia Z., Singh N., Kalitzeos A., Strauss R.W., Fujinami K., Michaelides M. Prospective Cohort Study of Childhood-Onset Stargardt Disease: Fundus Autofluorescence Imaging, Progression, Comparison with Adult-Onset Disease, and Disease Symmetry. Am. J. Ophthalmol. 2020;211:159–175. doi: 10.1016/j.ajo.2019.11.008. - DOI - PMC - PubMed
-
- Ávila-Fernández A., Cantalapiedra D., Aller E., Vallespín E., Aguirre-Lambán J., Blanco-Kelly F., Corton M., Riveiro-Álvarez R., Allikmets R., Trujillo-Tiebas M.J., et al. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol. Vis. 2010;16:2550–2558. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

