Amylin Protein Expression in the Rat Brain and Neuro-2a Cells
- PMID: 35457166
- PMCID: PMC9025265
- DOI: 10.3390/ijms23084348
Amylin Protein Expression in the Rat Brain and Neuro-2a Cells
Abstract
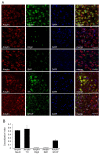
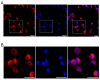
The localization and expression of amylin protein in the rodent brain and mouse neuroblastoma Neuro-2a (N2a) are less widely known. Thus, this study investigated the expression distribution of amylin in the rat brain and N2a treated with steroid hormones. Amylin protein was identified in the olfactory bulb, cerebral cortex, dentate gyrus, thalamus, hypothalamus, ventral tegmental area (VTA), cerebellum, and brain stem in the rat brain. Additionally, the amylin protein was localized with the mature neurons of the cerebral cortex and dopaminergic neurons of the VTA. Progesterone (P4) and dexamethasone (Dex) significantly decreased, and 17β-estradiol (E2) increased the amylin protein level in the cerebral cortex. The P4 receptor antagonist RU486 significantly influenced the effects of P4 and Dex, and the E2 receptor antagonist ICI 182,780 slightly changed E2's effect. Amylin protein expression was significantly reduced in the VTA by P4 and Dex, and its expression was changed only following P4 plus RU486 treatment. It was confirmed for the first time that amylin protein is strongly expressed in the cytoplasm in N2a cells using immunofluorescent staining. P4 increased the levels of amylin, and RU486 treatment decreased them. Dex significantly increased the levels of amylin protein. RU486 treatment reversed the effects of Dex. Therefore, amylin protein is expressed in the cerebral cortex neurons and dopaminergic neurons of the VTA of the immature rat brain. P4 and Dex influence the expression of amylin protein in the rat brain and N2a cells.
Keywords: 17β-estradiol; Neuro-2a; amylin; dexamethasone; progesterone; rat brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Westermark P., Wernstedt C., Wilander E., Hayden D.W., O’Brien T.D., Johnson K.H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl. Acad. Sci. USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

