Nutrient Sensing and Biofilm Modulation: The Example of L-arginine in Pseudomonas
- PMID: 35457206
- PMCID: PMC9028604
- DOI: 10.3390/ijms23084386
Nutrient Sensing and Biofilm Modulation: The Example of L-arginine in Pseudomonas
Abstract
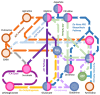
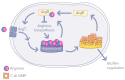
Bacterial biofilm represents a multicellular community embedded within an extracellular matrix attached to a surface. This lifestyle confers to bacterial cells protection against hostile environments, such as antibiotic treatment and host immune response in case of infections. The Pseudomonas genus is characterised by species producing strong biofilms difficult to be eradicated and by an extraordinary metabolic versatility which may support energy and carbon/nitrogen assimilation under multiple environmental conditions. Nutrient availability can be perceived by a Pseudomonas biofilm which, in turn, readapts its metabolism to finally tune its own formation and dispersion. A growing number of papers is now focusing on the mechanism of nutrient perception as a possible strategy to weaken the biofilm barrier by environmental cues. One of the most important nutrients is amino acid L-arginine, a crucial metabolite sustaining bacterial growth both as a carbon and a nitrogen source. Under low-oxygen conditions, L-arginine may also serve for ATP production, thus allowing bacteria to survive in anaerobic environments. L-arginine has been associated with biofilms, virulence, and antibiotic resistance. L-arginine is also a key precursor of regulatory molecules such as polyamines, whose involvement in biofilm homeostasis is reported. Given the biomedical and biotechnological relevance of biofilm control, the state of the art on the effects mediated by the L-arginine nutrient on biofilm modulation is presented, with a special focus on the Pseudomonas biofilm. Possible biotechnological and biomedical applications are also discussed.
Keywords: ArgR; Pseudomonas; RmcA; arginine; biofilm; c-di-GMP; metabolism; nutrients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- RM120172A7AD98EB to SR and AR12117A63EE6037 to CSR/Sapienza University of Rome
- PID2019-109372GB-I00/MCIN/AEI/10.13039/501100011033
- ERDF A way of making Europe/European Union
- Sensing arginine through the Venus Fly Trap domain to control c-di-GMP levels in Pseudomonas aeruginosa: molecular mechanism and metabolic effects of signal transduction./Istituto Pasteur Italia - Fondazione Cenci Bolognetti
LinkOut - more resources
Full Text Sources

