Neurodevelopmental Disorders Associated with PSD-95 and Its Interaction Partners
- PMID: 35457207
- PMCID: PMC9025546
- DOI: 10.3390/ijms23084390
Neurodevelopmental Disorders Associated with PSD-95 and Its Interaction Partners
Abstract
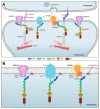
The postsynaptic density (PSD) is a massive protein complex, critical for synaptic strength and plasticity in excitatory neurons. Here, the scaffolding protein PSD-95 plays a crucial role as it organizes key PSD components essential for synaptic signaling, development, and survival. Recently, variants in DLG4 encoding PSD-95 were found to cause a neurodevelopmental disorder with a variety of clinical features including intellectual disability, developmental delay, and epilepsy. Genetic variants in several of the interaction partners of PSD-95 are associated with similar phenotypes, suggesting that deficient PSD-95 may affect the interaction partners, explaining the overlapping symptoms. Here, we review the transmembrane interaction partners of PSD-95 and their association with neurodevelopmental disorders. We assess how the structural changes induced by DLG4 missense variants may disrupt or alter such protein-protein interactions, and we argue that the pathological effect of DLG4 variants is, at least partly, exerted indirectly through interaction partners of PSD-95. This review presents a direction for functional studies to elucidate the pathogenic mechanism of deficient PSD-95, providing clues for therapeutic strategies.
Keywords: DLG4; clinical genetics; epilepsy; excitatory synapses; glutamate signaling; intellectual disability; postsynaptic density; synaptopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

