Anti-Inflammatory and Neuroprotective Mechanisms of GTS-21, an α7 Nicotinic Acetylcholine Receptor Agonist, in Neuroinflammation and Parkinson's Disease Mouse Models
- PMID: 35457238
- PMCID: PMC9026703
- DOI: 10.3390/ijms23084420
Anti-Inflammatory and Neuroprotective Mechanisms of GTS-21, an α7 Nicotinic Acetylcholine Receptor Agonist, in Neuroinflammation and Parkinson's Disease Mouse Models
Abstract
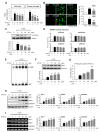
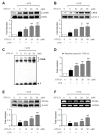
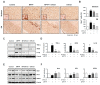
Neuroinflammation is crucial in the progression of neurodegenerative diseases. Thus, controlling neuroinflammation has been proposed as an important therapeutic strategy for neurodegenerative disease. In the present study, we examined the anti-inflammatory and neuroprotective effects of GTS-21, a selective α7 nicotinic acetylcholine receptor (α7 nAChR) agonist, in neuroinflammation and Parkinson's disease (PD) mouse models. GTS-21 inhibited the expression of inducible nitric oxide synthase (iNOS) and proinflammatory cytokines in lipopolysaccharide (LPS)-stimulated BV2 microglial cells and primary microglia. Further research revealed that GTS-21 has anti-inflammatory properties by inhibiting PI3K/Akt, NF-κB, and upregulating AMPK, Nrf2, CREB, and PPARγ signals. The effects of GTS-21 on these pro-/anti-inflammatory signaling molecules were reversed by treatment with an α7 nAChR antagonist, suggesting that the anti-inflammatory effects of GTS-21 are mediated through α7 nAChR activation. The anti-inflammatory and neuroprotective properties of GTS-21 were then confirmed in LPS-induced systemic inflammation and MPTP-induced PD model mice. In LPS-injected mouse brains, GTS-21 reduced microglial activation and production of proinflammatory markers. Furthermore, in the brains of MPTP-injected mice, GTS-21 restored locomotor activity and dopaminergic neuronal cell death while inhibiting microglial activation and pro-inflammatory gene expression. These findings suggest that GTS-21 has therapeutic potential in neuroinflammatory and neurodegenerative diseases such as PD.
Keywords: GTS-21; Parkinson’s disease; microglia; molecular mechanism; neuroinflammation; α7 nAChR agonist.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Catalin B., Cupido A., Iancau M., Albu C.V., Kirchhoff F. Microglia: First responders in the central nervous system. Rom. J. Morphol. Embryol. 2013;54:467–472. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

