Synthesis of Polyaniline Supported CdS/CdS-ZnS/CdS-TiO2 Nanocomposite for Efficient Photocatalytic Applications
- PMID: 35458061
- PMCID: PMC9032629
- DOI: 10.3390/nano12081355
Synthesis of Polyaniline Supported CdS/CdS-ZnS/CdS-TiO2 Nanocomposite for Efficient Photocatalytic Applications
Abstract
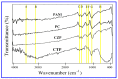
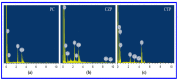
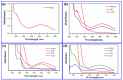
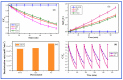
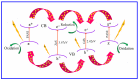
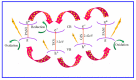
Photocatalytic degradation can be increased by improving photo-generated electrons and broadening the region of light absorption through conductive polymers. In that view, we have synthesized Polyaniline (PANI) with CdS, CdS-ZnS, and CdS-TiO2 nanocomposites using the chemical precipitation method, characterized and verified for the photo-degradation of Acid blue-29 dye. This paper provides a methodical conception about in what way conductive polymers "PANI" enhances the performance rate of composite photocatalysts (CdS, CdS-ZnS and CdS-TiO2). The nanocomposites charge transfer, molar ratio, surface morphology, particle size, diffraction pattern, thermal stability, optical and recombination of photo-generated charge carrier properties were determined. The production of nanocomposites and their efficient photocatalytic capabilities were observed. The mechanism of photocatalysis involved with PC, CZP and CTP nanocomposites are well presented by suitable diagrams representing the exchange of electrons and protons among themselves with supported equations. We discovered that increasing the number of nanocomposites in the membranes boosted both photocatalytic activity and degradation rate. CdS-Zinc-PANI (CZP) and CdS-TiO2-PANI(CTP) nanocomposites show entrapment at the surface defects of Zinc and TiO2 nanoparticles due to the demolition of unfavorable electron kinetics, and by reducing the charge recombination, greater photocatalytic activity than CdS-PANI (CP) with the same nanoparticle loading was achieved. With repeated use, the photocatalysts' efficiency dropped very little, hinting that they may be used to remove organic pollutants from water. The photocatalytic activity of CZP and CTP photocatalytic membranes was greater when compared to CdS-PANI, which may be due to the good compatibility between CdS and Zinc and TiO2, as well efficient charge carrier separation. PANI can also increase the split-up of photo-excited charge carriers and extend the absorption zone when combined with these nanoparticles. As a result, the development of outrageous performance photocatalysts and their potential uses in ecological purification and solar power conversion has been facilitated. The novelty of this article is to present the degradation of AB-29 Dye using nanocomposites with polymers and study the enhanced degradation rate. Few studies have been carried out on polymer nanocomposites and their application in the degradation of AB-29 dyes and remediation of water purposes. Nanoparticle CdS is a very effective photocatalyst, commonly used for water purification along with nanoparticle ZnS and TiO2; but cadmium ion-leaching makes it ineffective for practical and commercial use. In the present work, we have reduced the leaching of hazardous cadmium ions by trapping them in a polyaniline matrix, hence making it suitable for commercial use. We have embedded ZnS and TiO2 along with CdS in a polyaniline matrix and compared their photocatalytic activity, stability, and reusability, proving our nano-composites suitable for commercial purposes with enhanced activities and stabilities, which is a novelty. All synthesized nanocomposites are active within the near-ultraviolet to deep infrared (i.e., 340-850 nm). This gives us full efficiency of the photocatalysts in the sunlight and further proves the commercial utility of our nanocomposites.
Keywords: Acid blue-29 dye; conducting polymer; nanoparticles; photocatalytic mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite.Sci Rep. 2022 Apr 6;12(1):5759. doi: 10.1038/s41598-022-09479-0. Sci Rep. 2022. PMID: 35388044 Free PMC article.
-
Visible light-induced photocatalytic degradation of Reactive Blue-19 over highly efficient polyaniline-TiO2 nanocomposite: a comparative study with solar and UV photocatalysis.Environ Sci Pollut Res Int. 2018 Feb;25(4):3731-3744. doi: 10.1007/s11356-017-0663-1. Epub 2017 Nov 22. Environ Sci Pollut Res Int. 2018. PMID: 29168135
-
Floating bed reactor for visible light induced photocatalytic degradation of Acid Yellow 17 using polyaniline-TiO2 nanocomposites immobilized on polystyrene cubes.Environ Sci Pollut Res Int. 2020 May;27(13):14441-14453. doi: 10.1007/s11356-020-07959-2. Epub 2020 Feb 18. Environ Sci Pollut Res Int. 2020. PMID: 32072418
-
Progress in photocatalytic degradation of industrial organic dye by utilising the silver doped titanium dioxide nanocomposite.Heliyon. 2024 Dec 5;10(24):e40998. doi: 10.1016/j.heliyon.2024.e40998. eCollection 2024 Dec 30. Heliyon. 2024. PMID: 39720083 Free PMC article. Review.
-
Investigation of TiO2/PPy nanocomposite for photocatalytic applications; synthesis, characterization, and combination with various substrates: a review.Environ Sci Pollut Res Int. 2024 Jun;31(30):42521-42546. doi: 10.1007/s11356-024-33893-8. Epub 2024 Jun 15. Environ Sci Pollut Res Int. 2024. PMID: 38878243 Review.
Cited by
-
Recent Advances in the Removal of Organic Dyes from Aqueous Media with Conducting Polymers, Polyaniline and Polypyrrole, and Their Composites.Polymers (Basel). 2022 Oct 10;14(19):4243. doi: 10.3390/polym14194243. Polymers (Basel). 2022. PMID: 36236189 Free PMC article. Review.
-
Green synthesis and multifaceted applications: challenges and innovations in carbon dot nanocomposites.Discov Nano. 2024 Dec 17;19(1):205. doi: 10.1186/s11671-024-04124-3. Discov Nano. 2024. PMID: 39681796 Free PMC article. Review.
-
Advances in the Use of Conducting Polymers for Healthcare Monitoring.Int J Mol Sci. 2024 Jan 26;25(3):1564. doi: 10.3390/ijms25031564. Int J Mol Sci. 2024. PMID: 38338846 Free PMC article. Review.
References
-
- Koysuren O., Koysuren H.N. Photocatalytic activity of polyaniline/Fe-doped TiO2 composites by in situ polymerization method. J. Macromol. Sci. Part A. 2019;56:267–276. doi: 10.1080/10601325.2019.1565548. - DOI
-
- Preeti S., Abdullah M.M., Ikram S. Role of Nanomaterials and their Applications as Photo-catalyst and Senors: A Review. Nano Res. Appl. 2016;2:1.
-
- Singh P., MAbdullah M., Sagadevan S., Kaur C., Ikram S. Highly sensitive ethanol sensor based on TiO2 nanoparticles and its photocatalyst activity. Opt.-Int. J. Light Electron Opt. 2019;182:512–518. doi: 10.1016/j.ijleo.2019.01.077. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

