Actions of Retinoic Acid in the Pathophysiology of HIV Infection
- PMID: 35458172
- PMCID: PMC9029687
- DOI: 10.3390/nu14081611
Actions of Retinoic Acid in the Pathophysiology of HIV Infection
Abstract
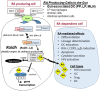
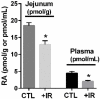
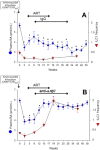
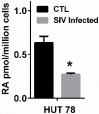
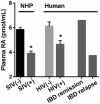
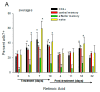
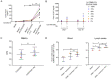
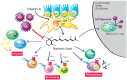
The vitamin A metabolite all-trans retinoic acid (RA) plays a key role in tissue homeostasis and mucosal immunity. RA is produced by gut-associated dendritic cells, which are among the first cells encountered by HIV. Acute HIV infection results in rapid reduction of RA levels and dysregulation of immune cell populations whose identities and function are largely controlled by RA. Here, we discuss the potential link between the roles played by RA in shaping intestinal immune responses and the manifestations and pathogenesis of HIV-associated enteropathy and similar conditions observed in SIV-infected non-human primate models. We also present data demonstrating the ability of RA to enhance the activation of replication-competent viral reservoirs from subjects on suppressive anti-retroviral therapy. The data suggest that retinoid supplementation may be a useful adjuvant for countering the pathologic condition of the gastro-intestinal tract associated with HIV infection and as part of a strategy for reactivating viral reservoirs as a means of depleting latent viral infection.
Keywords: HIV; immune regulation; mucosal immunity; retinoic acid; retinoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance.Mol Aspects Med. 2012 Feb;33(1):63-76. doi: 10.1016/j.mam.2011.11.001. Epub 2011 Nov 22. Mol Aspects Med. 2012. PMID: 22120429 Free PMC article. Review.
-
Retinoic acid imprints a mucosal-like phenotype on dendritic cells with an increased ability to fuel HIV-1 infection.J Immunol. 2015 Mar 1;194(5):2415-23. doi: 10.4049/jimmunol.1402623. Epub 2015 Jan 26. J Immunol. 2015. PMID: 25624458 Free PMC article.
-
Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes.J Pharmacol Exp Ther. 2000 Dec;295(3):979-85. J Pharmacol Exp Ther. 2000. PMID: 11082432
-
The regulation of HIV by retinoic acid correlates with cellular expression of the retinoic acid receptors.AIDS. 1994 Dec;8(12):1675-82. doi: 10.1097/00002030-199412000-00006. AIDS. 1994. PMID: 7888116
-
Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases.Mediators Inflamm. 2018 Aug 9;2018:3067126. doi: 10.1155/2018/3067126. eCollection 2018. Mediators Inflamm. 2018. PMID: 30158832 Free PMC article. Review.
Cited by
-
Retinoic acid enhances HIV-1 reverse transcription and transcription in macrophages via mTOR-modulated mechanisms.Cell Rep. 2024 Jul 23;43(7):114414. doi: 10.1016/j.celrep.2024.114414. Epub 2024 Jun 28. Cell Rep. 2024. PMID: 38943643 Free PMC article.
-
Dual role for microbial short-chain fatty acids in modifying SIV disease trajectory following anti-α4β7 antibody administration.Ann Med. 2024 Dec;56(1):2315224. doi: 10.1080/07853890.2024.2315224. Epub 2024 Feb 14. Ann Med. 2024. PMID: 38353210 Free PMC article.
-
Vedolizumab and ART in recent HIV-1 infection unveil the role of α4β7 in reservoir size.JCI Insight. 2024 Jul 9;9(16):e182312. doi: 10.1172/jci.insight.182312. JCI Insight. 2024. PMID: 38980725 Free PMC article. Clinical Trial.
-
Quantification of All-Trans Retinoic Acid and Cytokine Levels After Fungal, Viral and Bacterial Infections in the Lung.J Cell Mol Med. 2025 Mar;29(5):e70391. doi: 10.1111/jcmm.70391. J Cell Mol Med. 2025. PMID: 40031928 Free PMC article.
-
It's all in the gut: the central role of the gut and microbiome in preventing disease progression in simian immunodeficiency viruses infected African nonhuman primates.Curr Opin HIV AIDS. 2025 Mar 1;20(2):124-132. doi: 10.1097/COH.0000000000000911. Epub 2025 Jan 8. Curr Opin HIV AIDS. 2025. PMID: 39774258 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

