The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults
- PMID: 35458210
- PMCID: PMC9030734
- DOI: 10.3390/nu14081648
The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults
Abstract
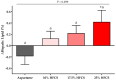
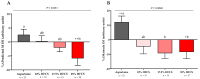
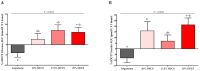
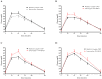
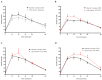
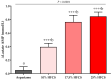
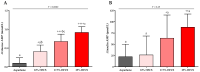
Increased hepatic lipid content and decreased insulin sensitivity have critical roles in the development of cardiometabolic diseases. Therefore, our objective was to investigate the dose-response effects of consuming high fructose corn syrup (HFCS)-sweetened beverages for two weeks on hepatic lipid content and insulin sensitivity in young (18-40 years) adults (BMI 18-35 kg/m2). In a parallel, double-blinded study, participants consumed three beverages/day providing 0% (aspartame: n = 23), 10% (n = 18), 17.5% (n = 16), or 25% (n = 28) daily energy requirements from HFCS. Magnetic resonance imaging for hepatic lipid content and oral glucose tolerance tests (OGTT) were conducted during 3.5-day inpatient visits at baseline and again at the end of a 15-day intervention. During the 12 intervening outpatient days participants consumed their usual diets with their assigned beverages. Significant linear dose-response effects were observed for increases of hepatic lipid content (p = 0.015) and glucose and insulin AUCs during OGTT (both p = 0.0004), and for decreases in the Matsuda (p = 0.0087) and Predicted M (p = 0.0027) indices of insulin sensitivity. These dose-response effects strengthen the mechanistic evidence implicating consumption of HFCS-sweetened beverages as a contributor to the metabolic dysregulation that increases risk for nonalcoholic fatty liver disease and type 2 diabetes.
Keywords: high-fructose corn syrup; insulin sensitivity; lactate; liver fat; sugar-sweetened beverages.
Conflict of interest statement
V.M. serves in the Advisory board of Alexion Pharmaceuticals. M.I.G. serves as Scientific Advisor to Yumi infant food and receives royalties for Sugarproof published by Penguin Random House. C.S. reports grants from GE, Siemens, Philips, Bayer, Foundation of NIH, Gilead, and Pfizer (grant is to UW-Madison; UCSD is a subcontract to UW-Madison); personal consultation fees from Blade, Boehringer, and Epigenomics; consultation under the auspices of the University to AMRA, BMS, Exact Sciences, GE Digital, IBM-Watson, and Pfizer; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, Takeda; royalties from Wolters Kluwer for educational material outside the submitted work; honoraria to the institution from Medscape for educational material outside the submitted work; ownership of stock options in Livivos; unpaid position in advisory board to Quantix Bio. All other authors have no conflict of interest to declare.
Figures

References
-
- Interntional Diabetes Federation IDF Diabetes Atlas: 10th Edition. [(accessed on 15 November 2021)]. Available online: http://www.diabetesatlas.org.
-
- Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., Fujii H., Wu Y., Kam L.Y., Ji F., et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. - DOI - PubMed

