Formulation and Evaluation of Transdermal Gel Containing Tacrolimus-Loaded Spanlastics: In Vitro, Ex Vivo and In Vivo Studies
- PMID: 35458277
- PMCID: PMC9024636
- DOI: 10.3390/polym14081528
Formulation and Evaluation of Transdermal Gel Containing Tacrolimus-Loaded Spanlastics: In Vitro, Ex Vivo and In Vivo Studies
Abstract
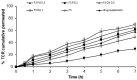
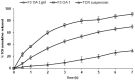
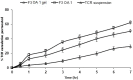
Our goal was to prepare Span 60-based elastic nanovesicles (spanlastics (SPLs)) of tacrolimus (TCR) using the adapted ethanol injection method, characterize them, and evaluate their ability to improve the transdermal permeation of the active substance. The impact of two different concentrations of penetration enhancers, namely, propylene glycol and oleic acid, on the entrapment efficiency, vesicle size, and zeta potential was assessed. Moreover, in vitro release through a semipermeable membrane and ex vivo penetration through hairless rat skin were performed. Morphological examination and pharmacokinetics were performed for one selected formulation (F3OA1). TCR-loaded SPLs were effectively formulated with two different concentrations of permeation enhancers, and the effect of these enhancers on their physicochemical properties differed in accordance with the concentration and kind of enhancer used. The results of in vitro release displayed a considerable (p < 0.05) enhancement compared to the suspension of the pure drug, and there was a correlation between the in vitro and ex vivo results. The selected TCR-loaded nanovesicles incorporated into a gel base showed appreciable advantages over the oral drug suspension and the TCR-loaded gel. Additionally, the pharmacokinetic parameters were significantly (p < 0.05) improved based on our findings. Moreover, the AUC0−7 ng·h/mL form F3 OA1 was 3.36-fold higher than that after the administration of the TCR oral suspension.
Keywords: drug permeation; penetration enhancers; spanlastics; tacrolimus; transdermal gel.
Conflict of interest statement
The authors hereby report that there are no conflicts of interest. The authors alone are accountable for the content and writing of this article.
Figures




Similar articles
-
Formulation of Polymers-Based Methotrexate Patches and Investigation of the Effect of Various Penetration Enhancers: In Vitro, Ex Vivo and In Vivo Characterization.Polymers (Basel). 2022 May 30;14(11):2211. doi: 10.3390/polym14112211. Polymers (Basel). 2022. PMID: 35683883 Free PMC article.
-
Penetration enhancer-containing spanlastics (PECSs) for transdermal delivery of haloperidol: in vitro characterization, ex vivo permeation and in vivo biodistribution studies.Drug Deliv. 2018 Nov;25(1):12-22. doi: 10.1080/10717544.2017.1410262. Drug Deliv. 2018. PMID: 29219628 Free PMC article.
-
Development and evaluation of surfactant-based elastic vesicular system for transdermal delivery of Cilostazole: ex-vivo permeation and histopathological evaluation studies.J Liposome Res. 2022 Jun;32(2):159-171. doi: 10.1080/08982104.2021.1918151. Epub 2021 May 10. J Liposome Res. 2022. PMID: 33970754
-
Fabrication of Thymoquinone and Ascorbic Acid-Loaded Spanlastics Gel for Hyperpigmentation: In Vitro Release, Cytotoxicity, and Skin Permeation Studies.Pharmaceutics. 2025 Jan 2;17(1):48. doi: 10.3390/pharmaceutics17010048. Pharmaceutics. 2025. PMID: 39861696 Free PMC article.
-
Microemulsions as transdermal drug delivery vehicles.Adv Colloid Interface Sci. 2006 Nov 16;123-126:369-85. doi: 10.1016/j.cis.2006.05.014. Epub 2006 Jul 14. Adv Colloid Interface Sci. 2006. PMID: 16843424 Review.
Cited by
-
Structural Characterization and Optimization of a Miconazole Oral Gel.Polymers (Basel). 2022 Nov 18;14(22):5011. doi: 10.3390/polym14225011. Polymers (Basel). 2022. PMID: 36433136 Free PMC article.
-
Overview of Spanlastics: A Groundbreaking Elastic Medication Delivery Device with Versatile Prospects for Administration via Various Routes.Curr Pharm Des. 2024;30(28):2206-2221. doi: 10.2174/0113816128313398240613063019. Curr Pharm Des. 2024. PMID: 38967069 Review.
-
Design, development, and preclinical evaluation of pirfenidone-loaded nanostructured lipid carriers for pulmonary delivery.Sci Rep. 2025 Apr 3;15(1):11390. doi: 10.1038/s41598-025-90910-7. Sci Rep. 2025. PMID: 40181013 Free PMC article.
-
Formulation, optimization and evaluation of ocular gel containing nebivolol Hcl-loaded ultradeformable spanlastics nanovesicles: In vitro and in vivo studies.Int J Pharm X. 2024 Jan 16;7:100228. doi: 10.1016/j.ijpx.2023.100228. eCollection 2024 Jun. Int J Pharm X. 2024. PMID: 38317829 Free PMC article.
-
Designing, Characterization, and Optimization of Nystatin Loaded Mucoadhesive Spanlastical Hard Candy Lozenges as a Treatment of Oral Candidiasis: An in-vivo Study on Rats.Drug Des Devel Ther. 2025 Apr 28;19:3293-3321. doi: 10.2147/DDDT.S504768. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40322040 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

