HBsAg Loss as a Treatment Endpoint for Chronic HBV Infection: HBV Cure
- PMID: 35458387
- PMCID: PMC9029793
- DOI: 10.3390/v14040657
HBsAg Loss as a Treatment Endpoint for Chronic HBV Infection: HBV Cure
Abstract
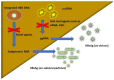
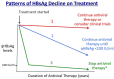
Despite the availability of effective vaccines and antiviral therapy over the past two to three decades, chronic hepatitis B virus (HBV) infection remains a major global health threat as a leading cause of cirrhosis and liver cancer. Functional HBV cure defined as hepatitis B surface antigen (HBsAg) loss and undetectable serum HBV DNA is associated with improved clinical outcomes in patients with chronic HBV infection. However, spontaneous loss of HBsAg is rare and occurs in only 1% of all HBsAg-positive individuals annually. Furthermore, the rate of functional cure with currently available antiviral therapy is even lower, <1% patients on treatment per year. Nonetheless, HBsAg loss has become the new target or therapeutic endpoint for antiviral treatment. Recently, there has been much excitement surrounding the development of novel antiviral agents such as small interfering RNA (siRNA), core assembly modulators (CAMs), nucleic acid polymers (NAPs) among others, which may be used in combination with nucleos(t)ide analogs and possibly immunomodulatory therapies to achieve functional cure in a significant proportion of patients with chronic hepatitis B. Novel assays with improved sensitivity for detection of very low levels of HBsAg and to determine the source of HBsAg production will also be required to measure efficacy of newer antiviral treatments for HBV cure. In this narrative review, we will define HBV cure, discuss various sources of HBsAg production, evaluate rates of HBsAg loss with current and future antiviral agents, review clinical factors associated with spontaneous HBsAg loss, and explore clinical implications of functional cure.
Keywords: HBV cure; antiviral therapy; hepatitis B surface antigen (HBsAg); hepatitis B virus (HBV).
Conflict of interest statement
S.F. serves as an advisor for Gilead Sciences, Janssen, Abbvie, Assembly Biosciences, Pfizer and Novo-Nordisk; receives research support from Gilead Sciences; speaks and teaches for Gilead Sciences, Abbvie and Lupin. M.M. declares no conflict of interest.
Figures
Comment in
-
Targeting Subviral Particles: A Critical Step in Achieving HBV Functional Cure but Where Are We with Current Agents in Clinical Development?Viruses. 2022 May 31;14(6):1193. doi: 10.3390/v14061193. Viruses. 2022. PMID: 35746664 Free PMC article.
References
-
- Razavi-Shearer D., Gamkrelidze I., Nguyen M.H., Chen D.S., Van Damme P., Abbas Z., Abdulla M., Abou Rached A., Adda D., Aho I., et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. - DOI - PubMed
-
- Chang M.-H., You S.-L., Chen C.-J., Liu C.-J., Lee C.-M., Lin S.-M., Chu H.-C., Wu T.-C., Yang S.-S., Kuo H.-S., et al. Decreased Incidence of Hepatocellular Carcinoma in Hepatitis B Vaccinees: A 20-Year Follow-up Study. JNCI J. Natl. Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. - DOI - PubMed
-
- Wasley A., Grytdal S., Gallagher K. Surveillance for acute viral hepatitis—United States, 2006. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2008;57:1–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

