VZV Infection of Primary Human Adrenal Cortical Cells Produces a Proinflammatory Environment without Cell Death
- PMID: 35458404
- PMCID: PMC9030771
- DOI: 10.3390/v14040674
VZV Infection of Primary Human Adrenal Cortical Cells Produces a Proinflammatory Environment without Cell Death
Abstract
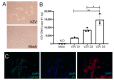
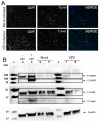
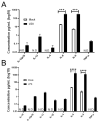
Virus infection of adrenal glands can disrupt secretion of mineralocorticoids, glucocorticoids, and sex hormones from the cortex and catecholamines from the medulla, leading to a constellation of symptoms such as fatigue, dizziness, weight loss, nausea, and muscle and joint pain. Specifically, varicella zoster virus (VZV) can produce bilateral adrenal hemorrhage and adrenal insufficiency during primary infection or following reactivation. However, the mechanisms by which VZV affects the adrenal glands are not well-characterized. Herein, we determined if primary human adrenal cortical cells (HAdCCs) infected with VZV support viral replication and produce a proinflammatory environment. Quantitative PCR showed VZV DNA increasing over time in HAdCCs, yet no cell death was seen at 3 days post-infection by TUNEL staining or Western Blot analysis with PARP and caspase 9 antibodies. Compared to conditioned supernatant from mock-infected cells, supernatant from VZV-infected cells contained significantly elevated IL-6, IL-8, IL-12p70, IL-13, IL-4, and TNF-α. Overall, VZV can productively infect adrenal cortical cells in the absence of cell death, suggesting that these cells may be a potential reservoir for ongoing viral replication and proinflammatory cytokine production, leading to chronic adrenalitis and dysfunction.
Keywords: adrenal cortex; adrenal glands; adrenalitis; cytokines; inflammation; varicella zoster virus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Varicella zoster virus differentially alters morphology and suppresses proinflammatory cytokines in primary human spinal cord and hippocampal astrocytes.J Neuroinflammation. 2018 Nov 15;15(1):318. doi: 10.1186/s12974-018-1360-9. J Neuroinflammation. 2018. PMID: 30442152 Free PMC article.
-
Histopathological Analysis of Adrenal Glands after Simian Varicella Virus Infection.Viruses. 2021 Jun 26;13(7):1245. doi: 10.3390/v13071245. Viruses. 2021. PMID: 34206909 Free PMC article.
-
Varicella Zoster Virus Induces Differential Cell-Type Specific Responses in Human Corneal Epithelial Cells and Keratocytes.Invest Ophthalmol Vis Sci. 2019 Feb 1;60(2):704-711. doi: 10.1167/iovs.18-25801. Invest Ophthalmol Vis Sci. 2019. PMID: 30786281 Free PMC article.
-
Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation.Viruses. 2019 Jan 26;11(2):103. doi: 10.3390/v11020103. Viruses. 2019. PMID: 30691086 Free PMC article. Review.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
Cited by
-
Viruses and Endocrine Diseases.Microorganisms. 2023 Jan 31;11(2):361. doi: 10.3390/microorganisms11020361. Microorganisms. 2023. PMID: 36838326 Free PMC article. Review.
-
Trigeminal Postherpetic Neuralgia: From Pathophysiology to Treatment.Curr Pain Headache Rep. 2024 Apr;28(4):295-306. doi: 10.1007/s11916-023-01209-z. Epub 2024 Jan 23. Curr Pain Headache Rep. 2024. PMID: 38261232 Free PMC article. Review.
-
Adrenal Abscesses: A Systematic Review of the Literature.J Clin Med. 2023 Jul 11;12(14):4601. doi: 10.3390/jcm12144601. J Clin Med. 2023. PMID: 37510716 Free PMC article. Review.
References
-
- Krajewska B. Two cases of hemorrhage into the suprarerenal glands in the course of varicella. Helv. Paediatr. Acta. 1960;15:71–73. - PubMed
-
- Rudkowski Z., Rutowski R. Zespół Waterhouse-Friderichsena w przebiegu ospy wietrznej u dziecka z toksoplazmoza [Wa-terhouse-Friderichsen syndrome during varicella in a child with toxoplasmosis] Pediatr. Pol. 1974;49:1023–1026. - PubMed
-
- Heitz A.F.N., Hofstee H.M.A., Gelinck L.B.S., Puylaert J.B. A rare case of Waterhouse- Friderichsen syndrome during primary varicella zoster. Neth. J. Med. 2017;75:351–353. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

