Management and Treatment of Patients with Chronic Hepatitis B: Towards Personalized Medicine
- PMID: 35458431
- PMCID: PMC9027850
- DOI: 10.3390/v14040701
Management and Treatment of Patients with Chronic Hepatitis B: Towards Personalized Medicine
Abstract
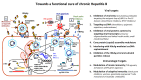
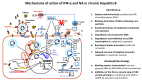
The currently available antiviral treatments (Peg-Interferon-α and Nucleos(t)ide Analogues, NA) for chronic hepatitis B (CHB) achieve a functional cure (serum HBsAg and HDV-DNA clearance) of HBV infection in a limited number of patients. Nevertheless, the continuous pharmacological suppression of viral replication by NA halts liver disease progression lowering the risk of HCC development and improving the survival. In the near future, to fully exploit the potential of old and new drugs for HBV treatment a personalized approach to the patients will be required according to an accurate definition of their virologic, immunologic and clinical profile.
Keywords: HBV; HBV functional cure; Interferon-α; antiviral treatment; chronic hepatitis B; nucleos(t)ide analogues.
Conflict of interest statement
M.R.B. Advisory Board and Speakers’ bureau: AbbVie, Gilead, Janssen and EISAI-MSD. The rest of the authors have no conflict of interest to declare.
Figures

References
-
- Cornberg M., Lok A.S., Terrault N.A., Zoulim F., 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty Guidance for design and endpoints of clinical trials in chronic hepatitis B—Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology. 2020;71:1070–1092. doi: 10.1002/hep.31030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

