mRNA- and Adenovirus-Based Vaccines against SARS-CoV-2 in HIV-Positive People
- PMID: 35458478
- PMCID: PMC9031858
- DOI: 10.3390/v14040748
mRNA- and Adenovirus-Based Vaccines against SARS-CoV-2 in HIV-Positive People
Abstract
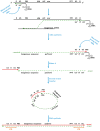
About two years have passed since the identification of SARS-CoV-2 in China. The rapid spread of this virus all over the world and its high transmissibility and pathogenicity in humans have resulted in a global pandemic. The negative impact of COVID-19 on health, society and the economy at the global level has pushed researchers and pharmaceutical companies to develop effective vaccines to fight SARS-CoV-2. Thanks to this collaborative effort, the first COVID-19 vaccine was developed in less than a year. Since then, several COVID-19 vaccines have been validated for use by the World Health Organization. Among these, mRNA- (BNT162b2 and mRNA1273) and adenovirus-based (ChAdOx1) vaccines were developed through the use of novel technologies. While all three of these vaccines have shown effectiveness against the COVID-19 disease and their immunogenicity was characterized in clinical trials in the general population, data on their efficacy and immunogenicity in people living with HIV (PLWH) are limited. In this review, we provide a description of the characteristics of mRNA- and adenovirus-based vaccines and of the immune response elicited in the general population by vaccination. Then we describe the use of these vaccines and their efficacy and immunogenicity in people living with HIV and we conclude with a discussion regarding some open questions concerning the use of mRNA- and adenovirus-based COVID-19 vaccines in PLWH.
Keywords: HIV infection; SARS-CoV-2; adenoviral vaccine; immune response; mRNA vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 22 February 2022)]. Available online: https://covid19.who.int.
-
- HAdV Working Group. [(accessed on 18 September 2021)]. Available online: http://hadvwg.gmu.edu/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

