West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage
- PMID: 35458486
- PMCID: PMC9027124
- DOI: 10.3390/v14040756
West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage
Abstract
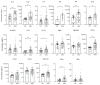
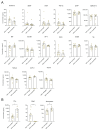
Among emerging arthropod-borne viruses (arbovirus), West Nile virus (WNV) is a flavivirus that can be associated with severe neuroinvasive infections in humans. In 2018, the European WNV epidemic resulted in over 2000 cases, representing the most important arboviral epidemic in the European continent. Characterization of inflammation and neuronal biomarkers released during WNV infection, especially in the context of neuronal impairments, could provide insight into the development of predictive tools that could be beneficial for patient outcomes. We first analyzed the inflammatory signature in the serum of WNV-infected mice and found increased concentrations of several inflammatory cytokines. We next analyzed serum and cerebrospinal-fluid (CSF) samples from a cohort of patients infected by WNV between 2018 and 2019 in Hungary to quantify a large panel of inflammatory cytokines and neurological factors. We found higher levels of inflammatory cytokines (e.g., IL4, IL6, and IL10) and neuronal factors (e.g., BDNF, GFAP, MIF, TDP-43) in the sera of WNV-infected patients with neuroinvasive disease. Furthermore, the serum inflammatory profile of these patients persisted for several weeks after initial infection, potentially leading to long-term sequelae and having a deleterious effect on brain neurovasculature. This work suggests that early signs of increased serum concentrations of inflammatory cytokines and neuronal factors could be a signature underlying the development of severe neurological impairments. Biomarkers could play an important role in patient monitoring to improve care and prevent undesirable outcomes.
Keywords: West Nile virus; flavivirus; neuroinvasive disease; peripheral biomarkers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

