Antiviral Efficacy of Molnupiravir for COVID-19 Treatment
- PMID: 35458493
- PMCID: PMC9031952
- DOI: 10.3390/v14040763
Antiviral Efficacy of Molnupiravir for COVID-19 Treatment
Abstract
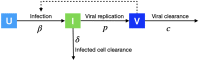
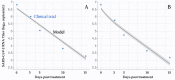
The ongoing global pandemic of COVID-19 poses unprecedented public health risks for governments and societies around the world, which have been exacerbated by the emergence of SARS-CoV-2 variants. Pharmaceutical interventions with high antiviral efficacy are expected to delay and contain the COVID-19 pandemic. Molnupiravir, as an oral antiviral prodrug, is active against SARS-CoV-2 and is now (23 February 2022) one of the seven widely-used coronavirus treatments. To estimate its antiviral efficacy of Molnupiravir, we built a granular mathematical within-host model. We find that the antiviral efficacy of Molnupiravir to stop the growth of the virus is 0.56 (95% CI: 0.49, 0.64), which could inhibit 56% of the replication of infected cells per day. There has been good progress in developing high-efficacy antiviral drugs that rapidly reduce viral load and may also reduce the infectiousness of treated cases if administered as early as possible.
Keywords: COVID-19; Molnupiravir; SARS-CoV-2; antiviral efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Molnupiravir and Its Antiviral Activity Against COVID-19.Front Immunol. 2022 Apr 4;13:855496. doi: 10.3389/fimmu.2022.855496. eCollection 2022. Front Immunol. 2022. PMID: 35444647 Free PMC article. Review.
-
Molnupiravir: A lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2-A narrative review.J Med Virol. 2022 Jul;94(7):3006-3016. doi: 10.1002/jmv.27730. Epub 2022 Apr 2. J Med Virol. 2022. PMID: 35315098 Free PMC article. Review.
-
Comprehensive review on molnupiravir in COVID-19: a novel promising antiviral to combat the pandemic.Future Microbiol. 2022 Mar;17(5):377-391. doi: 10.2217/fmb-2021-0252. Epub 2022 Feb 24. Future Microbiol. 2022. PMID: 35199608 Free PMC article. Review.
-
The effect of molnupiravir and nirmatrelvir on SARS-CoV-2 genome diversity in severe models of COVID-19.Microbiol Spectr. 2025 May 6;13(5):e0182924. doi: 10.1128/spectrum.01829-24. Epub 2025 Mar 25. Microbiol Spectr. 2025. PMID: 40130852 Free PMC article.
-
Efficacy of late-onset antiviral treatment in immunocompromised hosts with persistent SARS-CoV-2 infection.J Virol. 2024 Sep 17;98(9):e0090524. doi: 10.1128/jvi.00905-24. Epub 2024 Aug 29. J Virol. 2024. PMID: 39207133 Free PMC article.
Cited by
-
Molnupiravir When Used Alone Seems to Be Safe and Effective as Outpatient COVID-19 Therapy for Hemodialyzed Patients and Kidney Transplant Recipients.Viruses. 2022 Oct 9;14(10):2224. doi: 10.3390/v14102224. Viruses. 2022. PMID: 36298779 Free PMC article.
-
Assessing the Efficacy of Early Therapies against SARS-CoV-2 in Hematological Patients: A Real-Life Study from a COVID-19 Referral Centre in Northern Italy.J Clin Med. 2022 Dec 15;11(24):7452. doi: 10.3390/jcm11247452. J Clin Med. 2022. PMID: 36556068 Free PMC article.
-
Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial.EClinicalMedicine. 2024 Mar 14;70:102517. doi: 10.1016/j.eclinm.2024.102517. eCollection 2024 Apr. EClinicalMedicine. 2024. PMID: 38516100 Free PMC article.
-
Using virtual patient cohorts to uncover immune response differences in cancer and immunosuppressed COVID-19 patients.PLoS Comput Biol. 2025 Jun 9;21(6):e1013170. doi: 10.1371/journal.pcbi.1013170. eCollection 2025 Jun. PLoS Comput Biol. 2025. PMID: 40489562 Free PMC article.
-
Prevention and treatment strategies for kidney transplant recipients in the context of long-term existence of COVID-19.Front Med (Lausanne). 2024 Apr 3;11:1287836. doi: 10.3389/fmed.2024.1287836. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38633308 Free PMC article. Review.
References
-
- Oliver N., Lepri B., Sterly H., Lambiotte R., Deletaille S., De Nadai M., Letouzé E., Salah A.A., Benjamins R., Cattuto C., et al. Mobile Phone Data for Informing Public Health Actions across the COVID-19 Pandemic Life Cycle. Sci. Adv. 2020;6:eabc0764. doi: 10.1126/sciadv.abc0764. - DOI - PMC - PubMed
-
- Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. - DOI - PMC - PubMed
-
- Zimmer C., Wu K.J., Corum J., Kristoffersen M. Coronavirus Drug and Treatment Tracker. The New York Times. Oct 23, 2020. pp. 10–27.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

