Dermatitis during Spaceflight Associated with HSV-1 Reactivation
- PMID: 35458519
- PMCID: PMC9028032
- DOI: 10.3390/v14040789
Dermatitis during Spaceflight Associated with HSV-1 Reactivation
Abstract
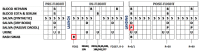
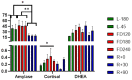
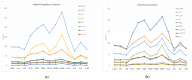
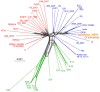
Human alpha herpesviruses herpes simplex virus (HSV-1) and varicella zoster virus (VZV) establish latency in various cranial nerve ganglia and often reactivate in response to stress-associated immune system dysregulation. Reactivation of Epstein Barr virus (EBV), VZV, HSV-1, and cytomegalovirus (CMV) is typically asymptomatic during spaceflight, though live/infectious virus has been recovered and the shedding rate increases with mission duration. The risk of clinical disease, therefore, may increase for astronauts assigned to extended missions (>180 days). Here, we report, for the first time, a case of HSV-1 skin rash (dermatitis) occurring during long-duration spaceflight. The astronaut reported persistent dermatitis during flight, which was treated onboard with oral antihistamines and topical/oral steroids. No HSV-1 DNA was detected in 6-month pre-mission saliva samples, but on flight day 82, a saliva and rash swab both yielded 4.8 copies/ng DNA and 5.3 × 104 copies/ng DNA, respectively. Post-mission saliva samples continued to have a high infectious HSV-1 load (1.67 × 107 copies/ng DNA). HSV-1 from both rash and saliva samples had 99.9% genotype homology. Additional physiological monitoring, including stress biomarkers (cortisol, dehydroepiandrosterone (DHEA), and salivary amylase), immune markers (adaptive regulatory and inflammatory plasma cytokines), and biochemical profile markers, including vitamin/mineral status and bone metabolism, are also presented for this case. These data highlight an atypical presentation of HSV-1 during spaceflight and underscore the importance of viral screening during clinical evaluations of in-flight dermatitis to determine viral etiology and guide treatment.
Keywords: dermatitis; herpes; immune depression; spaceflight; stress; viral reactivation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

