Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity
- PMID: 35458571
- PMCID: PMC9024455
- DOI: 10.3390/v14040841
Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity
Abstract
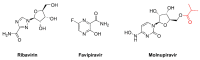
In RNA viruses, a small increase in their mutation rates can be sufficient to exceed their threshold of viability. Lethal mutagenesis is a therapeutic strategy based on the use of mutagens, driving viral populations to extinction. Extinction catastrophe can be experimentally induced by promutagenic nucleosides in cell culture models. The loss of HIV infectivity has been observed after passage in 5-hydroxydeoxycytidine or 5,6-dihydro-5-aza-2'-deoxycytidine while producing a two-fold increase in the viral mutation frequency. Among approved nucleoside analogs, experiments with polioviruses and other RNA viruses suggested that ribavirin can be mutagenic, although its mechanism of action is not clear. Favipiravir and molnupiravir exert an antiviral effect through lethal mutagenesis. Both drugs are broad-spectrum antiviral agents active against RNA viruses. Favipiravir incorporates into viral RNA, affecting the G→A and C→U transition rates. Molnupiravir (a prodrug of β-d-N4-hydroxycytidine) has been recently approved for the treatment of SARS-CoV-2 infection. Its triphosphate derivative can be incorporated into viral RNA and extended by the coronavirus RNA polymerase. Incorrect base pairing and inefficient extension by the polymerase promote mutagenesis by increasing the G→A and C→U transition frequencies. Despite having remarkable antiviral action and resilience to drug resistance, carcinogenic risks and genotoxicity are important concerns limiting their extended use in antiviral therapy.
Keywords: HIV; RNA polymerase; SARS-CoV-2; error catastrophe; favipiravir; lethal mutagenesis; molnupiravir; nucleoside analogs; ribavirin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Eigen M. From Strange Simplicity to Complex Familiarity: A Treatise on Matter, Information, Life and Thought. Oxford University Press; Cary, NC, USA: 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

