Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs
- PMID: 35458662
- PMCID: PMC9029618
- DOI: 10.3390/molecules27082468
Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs
Abstract
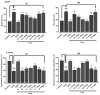
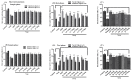
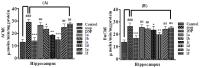
Alzheimer’s disease is an emerging health disorder associated with cognitive decline and memory loss. In this study, six curcumin analogs (1a−1f) were synthesized and screened for in vitro cholinesterase inhibitory potential. On the basis of promising results, they were further investigated for in vivo analysis using elevated plus maze (EPM), Y-maze, and novel object recognition (NOR) behavioral models. The binding mode of the synthesized compounds with the active sites of cholinesterases, and the involvement of the cholinergic system in brain hippocampus was determined. The synthesized curcumin analog 1d (p < 0.001, n = 6), and 1c (p < 0.01, n = 6) showed promising results by decreasing retention time in EPM, significantly increasing % SAP in Y-maze, while significantly (p < 0.001) enhancing the % discrimination index (DI) and the time exploring the novel objects in NORT mice behavioral models. A molecular docking study using MOE software was used for validation of the inhibition of cholinesterase(s). It has been indicated from the current research work that the synthesized curcumin analogs enhanced memory functions in mice models and could be used as valuable therapeutic molecules against neurodegenerative disorders. To determine their exact mechanism of action, further studies are suggested.
Keywords: Alzheimer’s disease; EPM; NORT; Y-maze; acetylcholinesterase; amnesia; butyrylcholinesterase; curcumin analogs; docking; hippocampus; scopolamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hussain H., Ahmad S., Wadood S., Shah A., Ghias M., Ullah A., Rahman S.U., Kamal Z., Khan F.A., Khan N.M., et al. Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules. 2021;26:7168. doi: 10.3390/molecules26237168. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

