Exogenous Melatonin Activating Nuclear Factor E2-Related Factor 2 (Nrf2) Pathway via Melatonin Receptor to Reduce Oxidative Stress and Apoptosis in Antler Mesenchymal Stem Cells
- PMID: 35458712
- PMCID: PMC9029981
- DOI: 10.3390/molecules27082515
Exogenous Melatonin Activating Nuclear Factor E2-Related Factor 2 (Nrf2) Pathway via Melatonin Receptor to Reduce Oxidative Stress and Apoptosis in Antler Mesenchymal Stem Cells
Abstract
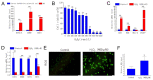
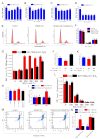
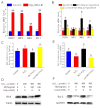
Antler growth depends on the proliferation and differentiation of mesenchymal stem cells (MSCs), and this process may be adversely affected by oxidative stress. Melatonin (MLT) has antioxidant functions, but its role in Cervidae remains largely unknown. In this article, flow cytometry, reactive oxygen species (ROS) identification, qPCR, and other methods were used to investigate the protective mechanism of MLT in H2O2-induced oxidative stress of antler MSCs. The results showed that MLT significantly increases cell viability by relieving the oxidative stress of antler MSCs. MLT inhibits cell apoptosis by protecting mitochondrial function. We blocked the melatonin receptor with luzindole (Luz) and found that the receptor blockade significantly increases H2O2-induced hyperoxide levels and causes significant inhibition of mitochondrial function. MLT treatment activates the nuclear factor E2-related factor 2 (Nrf2) antioxidant signaling pathway, up-regulates the expression of NAD(P)H quinone oxidoreductase 1 (NQO1) and other genes and it could inhibit apoptosis. In contrast, the melatonin receptor blockade down-regulates the expression of Nrf2 pathway-related genes, but significantly up-regulates the expression of apoptotic genes. It was indicated that MLT activates the Nrf2 pathway through the melatonin receptor and alleviates H2O2-induced oxidative stress and apoptosis in antler MSCs. This study provides a theoretical basis for further studying the oxidative stress and antioxidant process of antler MSCs and, thereby, increasing antler yields.
Keywords: MSCs; Nrf2 pathway; antler; apoptosis; melatonin; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gyurján I., Jr., Molnár A., Borsy A., Stéger V., Hackler L., Jr., Zomborszky Z., Papp P., Duda E., Deák F., Lakatos P., et al. Gene expression dynamics in deer antler: Mesenchymal differentiation toward chondrogenesis. Mol. Genet. Genom. 2007;277:221–235. doi: 10.1007/s00438-006-0190-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

