Stereoselective Synthesis of β-Glycinamide Ribonucleotide
- PMID: 35458726
- PMCID: PMC9024515
- DOI: 10.3390/molecules27082528
Stereoselective Synthesis of β-Glycinamide Ribonucleotide
Abstract
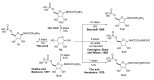
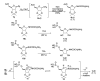
A diastereoselective synthesis of the β-anomer of glycinamide ribonucleotide (β-GAR) has been developed. The synthesis was accomplished in nine steps from D-ribose and occurred in 5% overall yield. The route provided material on the multi-milligram scale. The synthetic β-GAR formed was remarkably resistant to anomerization both in solution and as a solid.
Keywords: GART; stereoselective synthesis; β-GAR; β-glycinamide ribonucleotide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

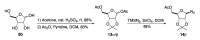
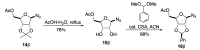
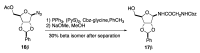



References
-
- Warren L., Flaks J.G. Single-carbon transfer reactions and purine biosynthesis. Fed. Proc. 1956;15:379.
-
- Warren L. Transformylation and purine biosynthesis. Fed. Proc. 1957;16:267.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

