Antagonistic network signature of motor function in Parkinson's disease revealed by connectome-based predictive modeling
- PMID: 35459232
- PMCID: PMC9033778
- DOI: 10.1038/s41531-022-00315-w
Antagonistic network signature of motor function in Parkinson's disease revealed by connectome-based predictive modeling
Abstract
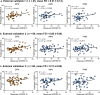
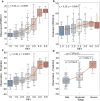
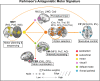
Motor impairment is a core clinical feature of Parkinson's disease (PD). Although the decoupled brain connectivity has been widely reported in previous neuroimaging studies, how the functional connectome is involved in motor dysfunction has not been well elucidated in PD patients. Here we developed a distributed brain signature by predicting clinical motor scores of PD patients across multicenter datasets (total n = 236). We decomposed the Pearson's correlation into accordance and discordance via a temporal discrete procedure, which can capture coupling and anti-coupling respectively. Using different profiles of functional connectivity, we trained candidate predictive models and tested them on independent and heterogeneous PD samples. We showed that the antagonistic model measured by discordance had the best sensitivity and generalizability in all validations and it was dubbed as Parkinson's antagonistic motor signature (PAMS). The PAMS was dominated by the subcortical, somatomotor, visual, cerebellum, default-mode, and frontoparietal networks, and the motor-visual stream accounted for the most part of predictive weights among network pairs. Additional stage-specific analysis showed that the predicted scores generated from the antagonistic model tended to be higher than the observed scores in the early course of PD, indicating that the functional signature may vary more sensitively with the neurodegenerative process than clinical behaviors. Together, these findings suggest that motor dysfunction of PD is represented as antagonistic interactions within multi-level brain systems. The signature shows great potential in the early motor evaluation and developing new therapeutic approaches for PD in the clinical realm.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
- No. 82072006/National Natural Science Foundation of China (National Science Foundation of China)
- No. 61906034/National Natural Science Foundation of China (National Science Foundation of China)
- 62036003/National Natural Science Foundation of China (National Science Foundation of China)
- 62173070/National Natural Science Foundation of China (National Science Foundation of China)
- 2021-YF05-01211-SN/Chengdu Science and Technology Bureau
LinkOut - more resources
Full Text Sources

