Antibody-mediated delivery of a viral MHC-I epitope into the cytosol of target tumor cells repurposes virus-specific CD8+ T cells for cancer immunotherapy
- PMID: 35459256
- PMCID: PMC9027861
- DOI: 10.1186/s12943-022-01574-0
Antibody-mediated delivery of a viral MHC-I epitope into the cytosol of target tumor cells repurposes virus-specific CD8+ T cells for cancer immunotherapy
Abstract
Background: Redirecting pre-existing virus-specific cytotoxic CD8+ T lymphocytes (CTLs) to tumors by simulating a viral infection of the tumor cells has great potential for cancer immunotherapy. However, this strategy is limited by lack of amenable method for viral antigen delivery into the cytosol of target tumors. Here, we addressed the limit by developing a CD8+ T cell epitope-delivering antibody, termed a TEDbody, which was engineered to deliver a viral MHC-I epitope peptide into the cytosol of target tumor cells by fusion with a tumor-specific cytosol-penetrating antibody.
Methods: To direct human cytomegalovirus (CMV)-specific CTLs against tumors, we designed a series of TEDbodies carrying various CMV pp65 antigen-derived peptides. CMV-specific CTLs from blood of CMV-seropositive healthy donors were expanded for use in in vitro and in vivo experiments. Comprehensive cellular assays were performed to determine the presentation mechanism of TEDbody-mediated CMV peptide-MHC-I complex (CMV-pMHCI) on the surface of target tumor cells and the recognition and lysis by CMV-specific CTLs. In vivo CMV-pMHCI presentation and antitumor efficacy of TEDbody were evaluated in immunodeficient mice bearing human tumors.
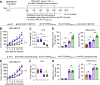
Results: TEDbody delivered the fused epitope peptides into target tumor cells to be intracellularly processed and surface displayed in the form of CMV-pMHCI, leading to disguise target tumor cells as virally infected cells for recognition and lysis by CMV-specific CTLs. When systemically injected into tumor-bearing immunodeficient mice, TEDbody efficiently marked tumor cells with CMV-pMHCI to augment the proliferation and cytotoxic property of tumor-infiltrated CMV-specific CTLs, resulting in significant inhibition of the in vivo tumor growth by redirecting adoptively transferred CMV-specific CTLs. Further, combination of TEDbody with anti-OX40 agonistic antibody substantially enhanced the in vivo antitumor activity.
Conclusion: Our study offers an effective technology for MHC-I antigen cytosolic delivery. TEDbody may thus have utility as a therapeutic cancer vaccine to redirect pre-existing anti-viral CTLs arising from previously exposed viral infections to attack tumors.
Keywords: Anti-viral cytotoxic T lymphocytes; Cytomegalovirus therapeutic cancer vaccine; Cytosol-penetrating antibody; MHC-I epitope cytosolic delivery; Peptide–MHC-I complex.
© 2022. The Author(s).
Conflict of interest statement
YSK, JAK, JK, SYL, and MJS are listed as inventors on pending patents (PCT/KR2021/001571, filed on 2 February 2021) related to the technology described in this work. All other coauthors they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

