A DNA vaccine targeting VEE virus delivered by needle-free jet-injection protects macaques against aerosol challenge
- PMID: 35459271
- PMCID: PMC9033795
- DOI: 10.1038/s41541-022-00469-x
A DNA vaccine targeting VEE virus delivered by needle-free jet-injection protects macaques against aerosol challenge
Abstract
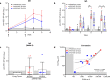
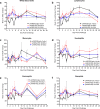
We have previously shown that DNA vaccines expressing codon optimized alphavirus envelope glycoprotein genes protect both mice and nonhuman primates from viral challenge when delivered by particle-mediated epidermal delivery (PMED) or intramuscular (IM) electroporation (EP). Another technology with fewer logistical drawbacks is disposable syringe jet injection (DSJI) devices developed by PharmaJet, Inc. These needle-free jet injection systems are spring-powered and capable of delivering vaccines either IM or into the dermis (ID). Here, we evaluated the immunogenicity of our Venezuelan equine encephalitis virus (VEEV) DNA vaccine delivered by either the IM- or ID-DSJI devices in nonhuman primates. The protective efficacy was assessed following aerosol challenge. We found that a prime and single boost by either the IM or ID route resulted in humoral and cellular immune responses that provided significant protection against disease and viremia. Although the ID route utilized one-fifth the DNA dose used in the IM route of vaccination, and the measured humoral and cellular immune responses trended lower, the level of protection was high and performed as well as the IM route for several clinical endpoints.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Department of the Army, the U.S. Department of Defense, or the U.S. Department of Health and Human Services or of the institutions and companies affiliated with the authors. Erin Spiegel is an employee of PharmaJet, Inc. The author acknowledges that there is a potential conflict of interest inherent in the publication of this manuscript and asserts that an effort to reduce or eliminate that conflict has been made where possible. There are no other conflicts of interest to report.
Figures


References
-
- Steele, K., et al. in Medical Aspects of Biological Warfare (ed Dembek Z. F.) Ch. Alphavirus encephalitis, 241-270 (Office of the Surgeon General, Department of the Army and US Army Medical Department Center and School, 2007).
-
- Rusnak JM, Dupuy LC, Niemuth NA, Glenn AM, Ward LA. Comparison of aerosol- and percutaneous-acquired venezuelan equine encephalitis in humans and nonhuman primates for suitability in predicting clinical efficacy under the animal rule. Comp. Med. 2018;68:380–395. doi: 10.30802/AALAS-CM-18-000027. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

