Vaccine effectiveness against onward transmission of SARS-CoV2-infection by variant of concern and time since vaccination, Belgian contact tracing, 2021
- PMID: 35459558
- PMCID: PMC9001203
- DOI: 10.1016/j.vaccine.2022.04.025
Vaccine effectiveness against onward transmission of SARS-CoV2-infection by variant of concern and time since vaccination, Belgian contact tracing, 2021
Abstract
Background: During the first half of 2021, we observed high vaccine effectiveness (VE) against SARS-CoV2-infection. The replacement of the alpha-'variant of concern' (VOC) by the delta-VOC and uncertainty about the time course of immunity called for a re-assessment.
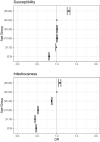
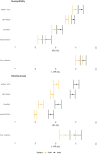
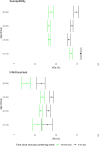
Methods: We estimated VE against transmission of infection (VET) from Belgian contact tracing data for high-risk exposure contacts between 26/01/2021 and 14/12/2021 by susceptibility (VEs) and infectiousness of breakthrough cases (VEi) for a complete schedule of Ad26.COV2.S, ChAdOx1, BNT162b2, mRNA-1273 as well as infection-acquired and hybrid immunity. We used a multilevel Bayesian model and adjusted for personal characteristics (age, sex, household), background exposure, calendar week, VOC and time since immunity conferring-event.
Findings: VET-estimates were higher for mRNA-vaccines, over 90%, compared to viral vector vaccines: 66% and 80% for Ad26COV2.S and ChAdOx1 respectively (Alpha, 0-50 days after vaccination). Delta was associated with a 40% increase in odds of transmission and a decrease of VEs (72-64%) and especially of VEi (71-46% for BNT162b2). Infection-acquired and hybrid immunity were less affected by Delta. Waning further reduced VET-estimates: from 81% to 63% for BNT162b2 (Delta, 150-200 days after vaccination). We observed lower initial VEi in the age group 65-84 years (32% vs 46% in the age group 45-64 years for BNT162b2) and faster waning. Hybrid immunity waned slower than vaccine-induced immunity.
Interpretation: VEi and VEs-estimates, while remaining significant, were reduced by Delta and waned over time. We observed faster waning in the oldest age group. We should seek to improve vaccine-induced protection in older persons and those vaccinated with viral-vector vaccines.
Keywords: Bayesian analysis; Contact Tracing; Covid19; Infection; Infectiousness; SARS-CoV2; Susceptibility; Transmission; Vaccine effectiveness.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

