JAK inhibitors and COVID-19
- PMID: 35459733
- PMCID: PMC9035837
- DOI: 10.1136/jitc-2021-002838
JAK inhibitors and COVID-19
Erratum in
-
Correction: JAK inhibitors and COVID-19.J Immunother Cancer. 2023 Apr;11(4):e002838corr1. doi: 10.1136/jitc-2021-002838corr1. J Immunother Cancer. 2023. PMID: 37117008 Free PMC article. No abstract available.
Abstract
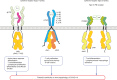
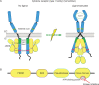
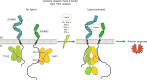
During SARS-CoV-2 infection, the innate immune response can be inhibited or delayed, and the subsequent persistent viral replication can induce emergency signals that may culminate in a cytokine storm contributing to the severe evolution of COVID-19. Cytokines are key regulators of the immune response and virus clearance, and, as such, are linked to the-possibly altered-response to the SARS-CoV-2. They act via a family of more than 40 transmembrane receptors that are coupled to one or several of the 4 Janus kinases (JAKs) coded by the human genome, namely JAK1, JAK2, JAK3, and TYK2. Once activated, JAKs act on pathways for either survival, proliferation, differentiation, immune regulation or, in the case of type I interferons, antiviral and antiproliferative effects. Studies of graft-versus-host and systemic rheumatic diseases indicated that JAK inhibitors (JAKi) exert immunosuppressive effects that are non-redundant with those of corticotherapy. Therefore, they hold the potential to cut-off pathological reactions in COVID-19. Significant clinical experience already exists with several JAKi in COVID-19, such as baricitinib, ruxolitinib, tofacitinib, and nezulcitinib, which were suggested by a meta-analysis (Patoulias et al.) to exert a benefit in terms of risk reduction concerning major outcomes when added to standard of care in patients with COVID-19. Yet, only baricitinib is recommended in first line for severe COVID-19 treatment by the WHO, as it is the only JAKi that has proven efficient to reduce mortality in individual randomized clinical trials (RCT), especially the Adaptive COVID-19 Treatment Trial (ACTT-2) and COV-BARRIER phase 3 trials. As for secondary effects of JAKi treatment, the main caution with baricitinib consists in the induced immunosuppression as long-term side effects should not be an issue in patients treated for COVID-19.We discuss whether a class effect of JAKi may be emerging in COVID-19 treatment, although at the moment the convincing data are for baricitinib only. Given the key role of JAK1 in both type I IFN action and signaling by cytokines involved in pathogenic effects, establishing the precise timing of treatment will be very important in future trials, along with the control of viral replication by associating antiviral molecules.
Keywords: COVID-19; autoimmunity; cytokines; hematologic neoplasms; therapies, investigational.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SC is cofounder of MyeloPro Diagnostics and Research, Vienna and of AlsaTech, Boston, MA, companies that aim to treat myeloproliferative neoplasms (MyeloPro) and neurodegenerative diseases (AlsaTech). PL is an employee of Incyte which developed ruxolitinib and owns stock of Incyte.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
