The dopaminergic control of Cushing's syndrome
- PMID: 35460460
- PMCID: PMC9184412
- DOI: 10.1007/s40618-021-01661-x
The dopaminergic control of Cushing's syndrome
Abstract
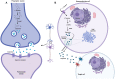
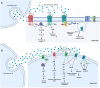
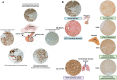
Cushing's Syndrome (CS), or chronic endogenous hypercortisolism, is a rare and serious disease due to corticotroph pituitary (Cushing's disease, CD) and extra-pituitary (ectopic CS) tumours overproducing ACTH, or cortisol-secreting adrenal tumours or lesions (adrenal CS). The first-line treatment for CS is represented by the surgical removal of the responsible tumour, but surgery might be unfeasible or ineffective and medical treatment can be required in a relevant percentage of patients with CS, especially CD and ectopic CS. Corticotroph pituitary and extra-pituitary tumours, as well as adrenal tumours and lesions responsible for CS express dopamine receptors (DRs), which have been found to mediate inhibition of hormone secretion and/or cell proliferation in experimental setting, suggesting that dopaminergic system, particularly DRs, might represent a target for the treatment of CS. Dopamine agonists (DAs), particularly cabergoline (CAB), are currently used as off-label treatment for CD, the most common form of CS, demonstrating efficacy in controlling hormone secretion and tumour growth in a relevant number of cases, with the improvement of clinical picture, and displaying good safety profile. Therefore, CAB may be considered a reasonable alternative treatment for persistent or recurrent CD after pituitary surgery failure, but occasionally also before pituitary surgery, as adjuvant treatment, or even instead of pituitary surgery as first-line treatment in case of surgery contraindications or refusal. A certain beneficial effect of CAB has been also reported in ectopic CS. However, the role of DAs in the clinical management of the different types of CS requires further evaluations.
Keywords: Cabergoline; Cushing’s disease; Cushing’s syndrome; Dopamine; Dopaminergic system.
© 2022. The Author(s).
Conflict of interest statement
R.P. has been Principal Investigator of Clinical and/or Translational Research Studies for Novartis, HRA Pharma, Ipsen, Shire, Corcept Therapeutics, Cortendo AB-Strongbridge Biopharma, Janssen Cilag, Camurus and Pfizer; Co-investigator of Research Studies for Pfizer; received research grants from Novartis, Pfizer, Ipsen, HRA Pharma, Shire, IBSA, Strongbridge Biopharma; has been an occasional consultant for Novartis, Ipsen, Pfizer, Shire, HRA Pharma, Cortendo AB-Strongbridge Biopharma, Ferring, Recordati Rare Disease, Corcept Therapeutics, Crinetics Pharmaceuticals, ARH Healthcare, Biohealth Italia, Damor Farmaceutici; and has received fees and honoraria for presentations from Novartis, Shire, Pfizer and Recordati beyond the confines of this work. C.P. received research grants from Corcept Therapeutics. C.S. has been occasional consultant for Shire and Ipsen. A.C. has been Principal Investigator of Research Studies for Novartis, Ipsen, Pfizer, Lilly, Merck and Novo Nordisk; consultant for Novartis, Ipsen, Pfizer, and received honoraria from Novartis, Ipsen and Pfizer beyond the confines of this work. M.C.D.M. has nothing to disclose.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

